Irinotecan/Cisplatin With Split-Course Thoracic Irradiation Set to Proceed to Phase II Study
TOKYO, JAPAN-The administration of irinotecan (Camptosar) 60 mg/m² and cisplatin (Platinol) 80 mg/m² with concurrent thoracic radiotherapy has been identified as the recommended dose for evaluation in a phase II study in patients with locally advanced non–small-cell lung cancer (NSCLC).
TOKYO, JAPANThe administration of irinotecan (Camptosar) 60 mg/m² and cisplatin (Platinol) 80 mg/m² with concurrent thoracic radiotherapy has been identified as the recommended dose for evaluation in a phase II study in patients with locally advanced nonsmall-cell lung cancer (NSCLC).
Yoichi Nakamura, MD, and colleagues at the Japanese Red Cross Nagasaki Bomb Hospital in Nagasaki, Japan, conducted a phase I study to determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of the two drugs in combination with concurrent thoracic radiation treatment in patients with previously untreated and unresectable stage III NSCLC.
According to the study protocol, irinotecan was administered intravenously on days 1, 8, and 15, and cisplatin was administered intravenously on day 1. The cycle was repeated every 28 days.
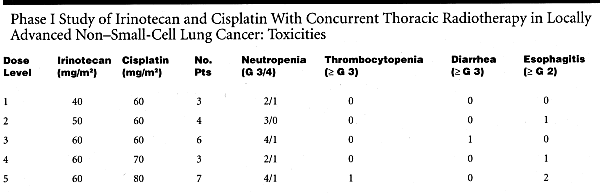
The initial dose was irinotecan 40 mg/m², and cisplatin 60 mg/m² (level 1), which was escalated to irinotecan 50 mg/m² and cisplatin 60 mg/m² (level 2), irinotecan 60 mg/m² and cisplatin 60 mg/m² (level 3), irinotecan 60 mg/m² and cisplatin 70 mg/m² (level 4), and irinotecan 60 mg/m² and cisplatin 80 mg/m² (level 5).
Standard thoracic radiotherapy of 2 Gy daily was started on day 2 of each chemotherapy cycle. A total of 24 Gy was administered in the first cycle and 26 to 36 Gy in the second cycle.
At least three patients were included at each dose level, with escalation to the next higher dose if no acute DLT was observed. If DLT developed in one of three patients, three additional patients were entered at the same dose level. The MTD was determined when greater than one third of patients experienced DLT at a given dose. No intrapatient dose escalation was performed.
Twenty-three of 24 patients entered in the trial were eligible for evaluation. The median age of the study participants was 62 years; most were men. Five patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0; 19 had a performance status of 1. Seven patients had stage IIIA and 17 had stage IIIb disease.
One patient experienced DLT at level 3 (diarrhea) and another at level 5 (neutropenia), however the MTD could not be identified. (See table.) Dose escalation was stopped at level 5 because this dose level was the recommended dose of chemotherapy alone for NSCLC.
There was one complete response and 15 partial responses, resulting in an overall response rate of 69.6%. The median survival time was 360 days.
Dr. Nakamura said the results show that irinotecan and cisplatin with concurrent thoracic radiotherapy given on a split schedule is tolerable and that level 5 should be tested in a phase II study.