Judging multigene tests for breast cancer recurrence before the results are in
Mammaprint and Oncotype DX are on the market many years before results are due from the large multicenter studies that should clarify their roles. In the meantime, oncologists and their patients face uncertainties about their best uses. Reports at the ASCO breast cancer symposium may resolve a few of them.
We've come a long way since the discovery of the BRCA genes. For many patients with early breast cancer, life-and-death decisions are now informed by tests involving dozens of genes, which often lead their doctors to recommend overruling the latest guidelines.
One breast-cancer website cheers these tests as a "breakthrough of genomic medicine," and a prominent team of researchers in the field works at the Breakthrough Breast Cancer Research Unit. But like many so-called breakthroughs, this process of breaking through has littered the field with uncertainties.
Predictably, tests such as Oncotype DX (Genomic Health, Inc.) and Mammaprint (Agendia, Inc.) are on the market and in common use many years before results are due from the large multicenter studies that should clarify their roles. In the meantime, oncologists and their patients face many questions : Which is best in which settings? How do they relate to each other? What are the real implications of high risk, and can we figure out whose risk is so low that even the test itself is unnecessary?
Modifying MammaPrintcriteria
The two major gene-profiling tests for breast cancer differ in fundamental ways: Oncotype DX measures the expression of 21 genes using PCR, while MammaPrint analyzes messenger RNA signals for 70 genes. It requires fresh tissue samples, while Oncotype DX can use fixed tissue. Mammaprint can be used to test patients with any estrogen-receptor (ER) status, while Oncotype DX is approved only for ER+ patients.
At last May's annual ASCO meeting, the team from the Netherlands Cancer Institute that developed the Mammaprint test reported results from 1630 eligible breast tumor samples showing significant 10-year survival differences between those that tested high and low risk (for instance, 82% versus 91%, respectively, for those graded G1).
Earlier this month at the ASCO breast cancer symposium, researchers from Agendia, Inc.announced a new 80-gene subtyping kit that further stratifies the risk of patients classified as high-risk by the 70-gene Mammaprint panel. As to low-risk patients, they had such small odds of recurrence that adding chemotherapy "does not appear to be clinically meaningful," the Netherlands team said.
Predicting low recurrence odds
Is it possible to predict which patients are at such low risk that they can safely forego chemotherapy without actually doing the multigene tests? Studies have examined pathologic and clinical factors associated with high risk, but less is known about the other end of the spectrum. At the recent breast cancer symposium, a team from the Vermont Cancer Center and Maimonides Cancer Center in Brooklyn, NY, revealed the factors that correlate with low risk of recurrence on Oncotype DX.
A mluticenter study published two years ago in the Journal of Clinical Oncology found Oncotype DX a better predictor than clinicopathological variables at both low and high risk levels. But it did not include some of the measures that turned up as useful in this small study.
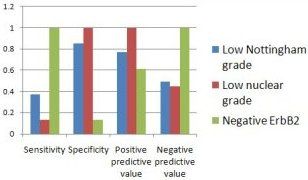
Retrospectively analyzing all 93 cases of early stage invasive breast cancer at the Vermont center for which molecular profiling was available between 2006 and 2009, they found that three factors--low Nottingham grade, low nuclear grade, and negative erbB2--predict a recurrence score below 19, which is defined as low risk on Oncotype DX. When evaluating multigene testing, they suggest, look at these indicators first.
Clinicopathologic Predictors of Low Recurrence Score
Source: Iberri DJ et al, ASCO Breast Cancer Symposium 2010
Not just lymph-node negative
Oncotype DX is designed for patients with ER-positive, lymph-node negative breast cancer, and guidelines from the NCCN and ASCO recommend it for clearly defined uses, such as type of tumor and type of treatment. But a report from Penn State Hershey Breast Center in Pennsylvania, described as the largest single-center experience to date using the 21-gene testfor breast cancer prognosis, finds that this may be too restrictive.
Of 205 patients since July 2007 who were willing or able to base treatment decisions on Oncotype DX results, more than half (106) accepted recommendations to change their therapy from that recommended by the 2005 NCCN guidelines. (Ten changed from endocrine therapy alone to chemo- plus endocrine, and the others decided to forgo chemotherapy in favor of endocrine treatment.)
Seventeen of these patients had 1 or 2 positive nodes, of whom 11 were treated with endocrine therapy alone based on the test results, and the researchers deem the test suitable for node-positive patients of this type. No patients have died of breast cancer during the study period, although one developed a contralateral new primary.
Refining an alternative to Oncotype DX
Oncotype DX was originally created to judge prognosis after tamoxifen therapy for ER-positive, node-negative patients. Two years ago, a team from the optomistically named Breakthrough Breast Cancer Research Unit at Kings College Hospital in London developed an alternative for similar patients who have not yet been treated. Since then, members of the same team have been expanding and amplifying the 14-gene metastasis score (MS). At the ASCO symposium they announced that the expanded version (the composite metastasis score, or cMS) is now more or less equivalent to Oncotype DX, although their gene panels are quite different.
Testing tissue samples collected by the Blumenthal Cancer Center in Charlotte, NC, the research team scored them all by both methods. Where Oncotype DX scored 29 as low risk, cMS scored 28 as low risk; 15 of 16 classified as high risk by Oncotype DX were deemed the same by the alternate test. (The cMS includes a multiplex assay for estrogen and progesterone receptors and HER2 as well as the 14 genes in the original metastasis score.)
Breast cancer genes: A moving target
Definitive results about these two multigene tests are forthcoming from multicenter studies, but oncologists have to wait a while. TailoRx, a huge multicenter study testing the best treatment outcomes after Oncotype DX results that score in the intermediate range, will complete data collection in four years. MindACT, a randomized European study comparing Mammaprint with clinical assessment, is due to complete data collection in 2019.
Neither of these trials compares the commercial tests with established but less-expensive methods, observe authors of a recent review of biomarkers in breast cancer. "We should not be misled by the seductiveness of the new," they add.
Doubtless new breast cancer genes--and the tests to detect them--will continue to emerge. As to the uncertainties about prognosis, therefore, there's actually no end in sight.