LUX-LUNG 3 and 4 Show Afatinib Effective in EGFR-mutated NSCLC
Two new studies show that afatinib, an orally bioavailable ERBB family blocker, could be effective in patients with non–small-cell lung cancer (NSCLC) who progressed on other therapies and in patients with advanced lung adenocarcinomas and EGFR mutations.
Two new studies show that afatinib, an orally bioavailable ERBB family blocker, could be effective in patients with non–small-cell lung cancer (NSCLC) who progressed on other therapies, and in patients with advanced lung adenocarcinomas and EGFR mutations.
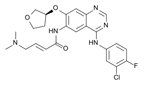
Chemical formula of afatinib
In the phase III LUX-LUNG 3 trial, Lecia V. Sequist, MD, MPH, of Harvard Medical School in Boston, and colleagues, randomized 345 patients with stage IIIB/IV lung adenocarcinoma and EGFR mutations to receive either afatinib 40 mg/day or up to 6 cycles of cisplatin plus pemetrexed chemotherapy. The median progression-free survival was 11.1 months for afatinib patients compared with 6.9 months for chemotherapy, for a hazard ratio (HR) of 0.58 (95% CI, 0.43–0.78; P = .001). Those with exon 19 deletions and L858R EGFR mutations (308 patients) fared even better with afatinib, with a progression-free survival of 13.6 vs 6.9 with chemotherapy for an HR of 0.47 (95% CI, 0.34–0.65; P = .001).
Afatinib fared significantly better than chemotherapy in female patients but not in male patients, and also did better in patients under the age of 65. Patients with baseline ECOG scores of 0 or 1 both saw significant improvements with afatinib vs chemotherapy.
The authors concluded that this and other research now strongly support the use of EGFR tyrosine kinase inhibitors (TKIs) as first-line treatment for patients with EGFR mutation–positive NSCLC. “EGFR testing should be tightly woven into lung cancer diagnostic workup algorithms worldwide,” they wrote. Further, about half of patients with the mutations will develop a resistance mutation after treatment with erlotinib or gefitinib; afatinib has shown activity against that particular mutation (T790M). The results of this study now suggest that afatinib should be considered as a standard option for this patient group.
LUX-LUNG 4
The phase II LUX-LUNG 4 trial gives further support to the idea that afatinib may be a good option for patients who develop that T790M resistance mutation following treatment with other TKIs. Researchers led by Nobuyuki Katakami, MD, PhD, of Kobe City Medical Center General Hospital in Kobe, Japan, treated 62 patients who were EGFR-mutation positive in the primary tumor with afatinib at 50 mg/day. Fifty-one of the patients (82.3%) qualified as having acquired resistance to erlotinib and/or gefitinib.
Of the 61 patients who were evaluable, five (8.2%) had a confirmed objective response to afatinib. The median progression-free survival was 4.4 months, with a median overall survival of 19 months. Among the afatinib-related adverse events, all patients experienced diarrhea and most (91.9%) had rash/acne; 29% of patients had an adverse event that led to discontinuation of the drug.
“The results of our trial demonstrated modest but noteworthy activity of afatinib in this difficult-to-treat population,” the authors wrote. They noted that further trials are now ongoing, including one phase IB trial testing the combination of afatinib and cetuximab in a small number of patients. “There is an increasing need to develop new molecular targeted agents that address the issue of resistance to erlotinib and/or gefitinib in patients with NSCLC who initially respond to treatment and then subsequently progress.”