Management of Liver Metastases From Colorectal Cancer
The liver is a frequent site of metastatic colorectal disease. Over the past 20 years, improvements in systemic chemotherapy and surgical techniques have improved the survival of patients with hepatic metastases. For 4 decades, fluorouracil and leucovorin were the only drugs available to treat metastatic colorectal cancer, but several new drugs and a variety of novel regimens are now available. Further improvements in results have been seen with the delivery of chemotherapy via the hepatic artery. Surgical resection of liver metastases has been encouraged when possible, and recent advances in surgery such as portal vein embolization, have made liver resection a possibility for more patients. This review considers the timing and sequence of chemotherapy and surgery in this setting, as well as the roles of cryoablation, radiofrequency ablation, and radiation therapy.
The liver is a frequent site of metastatic colorectal disease. Over the past 20 years, improvements in systemic chemotherapy and surgical techniques have improved the survival of patients with hepatic metastases. For 4 decades, fluorouracil and leucovorin were the only drugs available to treat metastatic colorectal cancer, but several new drugs and a variety of novel regimens are now available. Further improvements in results have been seen with the delivery of chemotherapy via the hepatic artery. Surgical resection of liver metastases has been encouraged when possible, and recent advances in surgery such as portal vein embolization, have made liver resection a possibility for more patients. This review considers the timing and sequence of chemotherapy and surgery in this setting, as well as the roles of cryoablation, radiofrequency ablation, and radiation therapy.
The liver is a frequent site of metastatic disease, especially for cancers of the gastrointestinal tract. Since venous drainage from the colon and rectum flows via the portal vein to the liver,[1] it is not surprising that patients with colorectal cancer frequently develop liver metastases. Approximately 15% of patients will have liver metastases at the time of diagnosis, and another 60% of patients who develop metastatic disease will have metastases to the liver. For many years, the approach to patients with hepatic metastases was nihilistic. In the past 2 decades, improvements in systemic chemotherapy, in modalities to detect liver metastases, and in surgical techniques for hepatic resection, have improved the survival of these patients.
Systemic Chemotherapy
TABLE 1
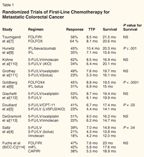
Randomized Trials of First-Line Chemotherapy for Metastatic Colorectal Cancer
For 4 decades, fluorouracil (5-FU) plus leucovorin (LV) were the only drugs available to treat metastatic colorectal cancer, yielding a response rate of 20% to 30% and a median survival of 11 to 12 months. A number of new agents are now available, including irinotecan (Camptosar), a topo-isomerase inhibitor, and oxaliplatin (Eloxatin), a platinum compound with in vivo and in vitro activity against colon cancer cell lines and the ability to synergize with 5-FU.[2,3]
Randomized trials using irinotecan with 5-FU/LV vs 5-FU/LV alone[4,5] produced an increase in response rate and survival (Table 1). When irinotecan/5-FU/LV (IFL) was compared to oxaliplatin plus 5-FU/LV (FOLFOX), the response rate was increased from 35% to 45%, and survival was increased from 15 to 19.5 months for the IFL and FOLFOX groups, respectively.[6] That said, 5-FU given by continuous infusion is more effective than bolus 5-FU; therefore, when 5-FU administration was changed to infusion (ie, FOLFIRI rather than IFL), the regimen of irinotecan/5-FU/LV became more effective and produced results similar to those seen with FOLFOX.[7]
TABLE 2

Second-Line Therapy: Systemic Therapy in Previously Treated Colorectal Cancer Patients
In the past few years, targeted agents have become available: bevacizumab (Avastin), a monoclonal antibody to vascular endothelial growth factor, and cetuximab (Erbitux), an antibody to epidermal growth factor receptor. The addition of bevacizumab to IFL[8] increased response rates and survival (35% to 45%, and 15.6 to 19.5 months, for IFL vs bevacizumab/IFL, respectively). For almost 4 decades, the 2-year survival for metastatic colorectal patients treated with 5-FU or 5-FU/LV was 25%; with these new agents, 2-year survival rates have increased to 30%-39%, with a marked improvement in overall survival (Table 1).
In the second-line setting, even with the new agents, results are less impressive. Irinotecan is associated with a response rate of 5% to 14% and a median survival of 9.9 months.[9] FOLFOX administered to irinotecan-refractory patients produces a 9.9% response rate, with a median survival of 9.8 months (Table 2).[10] After progression on irinotecan, cetuximab alone can produce a 10% response rate, which increases to 23% when this agent is combined with irinotecan.[11] Second-line bevacizumab combined with FOLFOX increased survival to 12 months from 10 months for FOLFOX alone (Table 2).[12]
Hepatic Arterial Infusional Chemotherapy
To further improve on results, adding direct liver perfusion with chemotherapy might be useful. The first trials compared hepatic arterial infusion (HAI) alone to systemic chemotherapy. The rationale for HAI is based on the following facts: (1) liver metastases are perfused almost exclusively by the hepatic artery, whereas the normal liver is perfused by the portal vein,[13] (2) certain drugs are largely extracted by the liver during the first pass, allowing for minimal systemic toxicity,[14] and (3) the liver is often the first and only site of metastatic disease.[15] Therefore, aggressive treatment of metastases confined to the liver by resection or hepatic infusion may yield prolonged survival for some patients.
Regional therapy can be delivered using a hepatic arterial port or a totally implantable pump. Early studies with catheters produced clotting and bleeding that did not allow for long-term hepatic infusion with reliable patency.[16] Studies in Europe still use the catheters rather than pumps, perhaps explaining the inferior results in the European trials.
Randomized Trials
TABLE 3
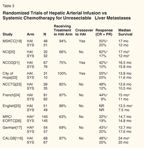
Randomized Trials of Hepatic Arterial Infusion vs Systemic Chemotherapy for Unresectable Liver Metastases
Ten randomized studies have compared HAI to systemic chemotherapy (Table 3).[17-26] Almost all the studies showed an increase in response rate. Only the English trial that used HAI 5-FU failed to increase response rate.
Why did the superior response rates seen with HAI not translate into improved survival in the earlier trials? Most of these trials were too small, and a number of them allowed crossover to HAI at the time of progression on systemic chemotherapy, potentially diluting statistical results that might have demonstrated a survival benefit. Two earlier European studies demonstrated an increase in survival with HAI, but appropriate systemic therapy was not always used.
Two recent European trials did not show an increase in survival-the first being the Medical Research Council and European Organization for Research and Treatment of Cancer (EORTC) study,[26] which randomized patients to HAI 5-FU/LV via a port rather than a pump (using 5-FU rather than floxuridine [FUDR]) vs systemic 5-FU/LV. In this study, 37% of the patients assigned to the HAI arm did not receive treatment and 29% had to stop treatment. No differences were seen in response rate at 12 weeks (22% vs 19%, for HAI and systemic therapy, respectively), and no differences were seen in toxicity, progression-free survival (PFS), or survival. The study was not analyzed to look at the patients who actually received treatment.
A German cooperative group[17] randomized patients to HAI FUDR, HAI 5-FU/LV, or systemic 5-FU/LV. Tumor response rates were 43.2%, 45%, and 19.7%, and development of extrahepatic disease was 40.5%, 12.5%, and 18.3% for the HAI FUDR, HAI 5-FU/LV, and systemic 5-FU/LV groups, respectively. Toxicity data indicated that 5-FU/LV therapy was much more toxic than FUDR. A port was used, rather than a pump, and the FUDR regimen was different from what was used in the American studies in that the dosage was reduced from 0.2 to 0.15 mg/kg/d after three cycles rather than adjusting for patient toxicity. The median survival was 12.7, 18.7, and 17.6 months for the HAI FUDR, HAI 5-FU/LV, and systemic 5-FU/LV groups, respectively. Only 66% of patients randomized to HAI FUDR were treated, but all were included in the survival analysis. Eight patients in the HAI FUDR group died before ever receiving treatment, perhaps explaining the very low survival with HAI FUDR.
The Cancer and Leukemia Group B (CALGB)[18] trial differs from the other HAI studies in that it included the use of dexamethasone in the HAI arm.[27] HAI FUDR, dexamethasone plus LV was compared to systemic bolus 5-FU/LV. No crossover was allowed. The HAI group had a significant increase in survival (24.4 months, vs 20 months in the systemic group, P = .0034). The time to hepatic progression was better in the HAI arm (9.8 vs 7.3 months, P = .034) but the time to extrahepatic progression was better in the systemic arm (14.8 vs 7.7 months in the HAI group, P < .029). The toxicities were as expected, with a significant increase in diarrhea and neutropenia for the systemic arm and a significant increase in biliary toxicity for the HAI arm (18% vs 0%). A quality-of-life assessment was performed as part of the study, and the HAI group experienced improvement in this parameter, as measured at 3 and 6 months.
Differences between the CALGB study[18] and the European studies might explain differences in the outcomes. The CALGB study used pumps instead of ports, and HAI therapy included FUDR with dexamethasone to decrease toxicity.[27] Survival was based on intent to treat in all three studies, and the actual number of patients treated was much lower in the European studies-66% in the German study and 63% in the English study-while it was 86% in the CALGB study. The CALGB study did demonstrate that regional therapy alone can improve survival over systemic 5-FU/LV with a survival similar to that seen with newer agents. Randomized studies of HAI therapy vs the new therapies have not been conducted. In the future, studies comparing HAI or HAI plus new agents vs the new agents alone would be appropriate.
Second-Line Therapy
TABLE 4

Hepatic Arterial Infusion in Previously Treated Patients: Second-Line Therapy
HAI-based therapy in patients refractory to systemic chemotherapy has produced much higher response rates in small single-institution studies (Table 4). A trial using HAI FUDR/LV/dexamethasone[28] in both chemotherapy-naive and previously treated patients produced response rates of 72% and 52%, and median survivals of 23 and 13.5 months for the chemotherapy-naive and previously treated patients, respectively. HAI FUDR/dexamethasone plus mitomycin administered through the pump sideport, produced a 70% response rate in previously treated patients, with a median survival of 19 months from the start of HAI therapy after progression on systemic 5-FU/LV.[29]
A phase I study of HAI FUDR combined with systemic irinotecan in previously treated patients (45% had previously received irinotecan) reported a response rate of 74%, a time to disease progression of 8.1 months, and a median survival of 20 months.[30] A total of 13 of the 16 patients with prior irinotecan exposure responded to this regimen. Systemic oxaliplatin plus 5-FU/LV or oxaliplatin plus irinotecan with concurrent HAI FUDR/dexamethasone in 36 previously treated patients (74% had received prior irinotecan) produced response rates of 86%, with a median survival of 36 months, and a 1-year survival of 80%.[31] These and other small studies (Table 4) suggest that a benefit may be derived from HAI and systemic therapy as second-line therapy, and randomized studies exploring the use of HAI plus systemic therapy vs new agents alone in the second-line setting should be performed.
Toxicity of Hepatic Arterial FUDR Infusion
The most common problems with HAI therapy are hepatic toxicity and gastric ulcerations. Myelosuppression, nausea, vomiting, and diarrhea do not occur with HAI FUDR. If diarrhea does occur, shunting to the bowel should be suspected.[32] Clinically, biliary toxicity is manifested as elevations of aspartate transaminase, alkaline phosphatase, and bilirubin.[28] In early stages of the disease, hepatic enzyme elevations will return to normal when the drug is withdrawn and the patient is given a rest period, whereas in more advanced cases, it does not resolve. Therefore, careful monitoring of liver function tests is necessary to avoid this toxicity. The bile ducts derive their blood supply almost exclusively from the hepatic artery,[33] and thus are also perfused with high doses of chemotherapy during HAI treatment. HAI 5-FU causes fewer biliary problems and more arteritis.[34]
In patients who develop jaundice, an endoscopic retrograde cholangiopancreatogram (ERCP) may demonstrate lesions resembling idiopathic sclerosing cholangitis in 5% to 29% of patients treated. The strictures may be focal and present at the hepatic duct bifurcation, and therefore drainage procedures either by ERCP or by transhepatic cholangiogram may be helpful. Duct obstruction from metastases or bile duct strictures from surgery can also be causes of elevated bilirubin and must be ruled out before concluding that the elevation in liver function tests is due to HAI therapy.
Liver Resection
Since autopsy series show that in one-third of patients dying from colorectal cancer, the liver is the only site of metastatic disease, surgical resection of liver metastases has been encouraged when possible. More than 20 large series show that liver resections are possible with reasonable mortality and morbidity, producing an average 5-year survival of about 30%.[35-41]
No randomized studies of liver resection vs systemic chemotherapy have been performed. Historically, however, the median survival for patients with liver metastases is about 6 to 12 months. Even with the newer therapies, the 2-year survival rate is generally less than 40%, and this drops rapidly at 3 and 4 years. In contrast, patients with liver metastases who have undergone resection generally have a 2-year survival of around 60% to 70% and a 5-year survival of 25% to 30%.
Surgical Advances
Recent advances in surgery have made liver resection a possibility for more patients. One technique, portal vein embolization,[42] removes the blood supply to the affected liver and thereby induces hypertrophy of the nondiseased portion that will remain after liver resection. If small remnant parts of the liver can undergo hypertrophy, larger resections of diseased areas can be done. The use of intraoperative ultrasonography allows the surgeon to find lesions missed by other modalities and also helps define the relationship of the tumor to hepatic vasculature.[43] Improved vascular clamping techniques,[44] controlled anatomic resection,[45] and other new strategies are outlined in a review by Khatri et al.[46] The use of radiofrequency ablation to small lesions in the remaining liver also extends liver resection options to more patients. Some reports have stated that even patients with positive nodes or small lung metastases can undergo liver resection with better outcomes than those seen with chemotherapy alone,[46] but these data are not widely accepted.
Patient Selection
There is no consensus for determining which patients are suitable for resection. A computer program (the OncoSurge model) was developed to aid in selecting these patients.[47] A panel of 16 experts from oncology, radiology, and liver surgery developed hypothetical patient profiles to decide when resection should be done first, chemotherapy should be done first followed by possible resection, or resection should be absolutely contraindicated. This consensus panel decided that absolute contraindications were unresectable extrahepatic disease, more than 70% liver involvement, liver failure, and being surgically unfit. Factors that did not influence treatment strategy were age, primary tumor stage, timing of metastasis detection, past blood transfusion, liver resection type, preresection carcinoembryonic antigen (CEA), or previous hepatectomy. They also suggested that patients with bilobar disease or more than four metastases receive resection only after tumors were shrunk with chemotherapy.
Prognostic Indicators
A number of published studies have attempted to identify prognostic factors associated with poorer outcomes, and two large series have developed a scoring system.[48,49] At Memorial Sloan-Kettering Cancer Center (MSKCC), for the 1,001 patients undergoing liver resection, the most important factors adversely affecting survival were size of tumor (> 5 cm), disease-free interval (< 12 months), number of tumors (> 1), lymph node-positive primary, and preoperative CEA (> 200 ng/mL). Giving 1 point for each of these factors, a score was obtained that correlated with survival. For scores of 0, 1-2, 3, and 4-5, the predicted 5-year survival rates were 60%, 42%, 20%, and 18%, respectively.[48]
Nordlinger et al[49] reviewed data on 1,568 patients who underwent liver resection for colorectal metastases. The 2- and 5-year survival rates of 64% and 28% were affected by age, size of the largest metastases, CEA level, stage of the primary, disease-free interval, number of liver nodules, and resection margin. Giving 1 point to each of these risk factors, the investigators calculated scores that divided the patients into three risk groups: 0-2, 3-4, and 5-7 with corresponding 2-year survival rates of 79%, 60%, and 43%, respectively.
The Liver Met Survey is an international Internet-based registry that includes 2,122 patients. In a recent report on this registry, the overall 5- and 10-year survival rates were 42% and 26%. The 5-year survival rates for three or fewer vs more than three nodules were 48% vs 24% (P = .0001). Another factor that affected survival was whether the tumor was unilateral or bilateral. In this review, preoperative chemotherapy did not benefit patients with solitary metastases (5-year survival rate: 45% vs 58%), whereas for patients with more than five metastases, the 5-year survival rate was 22% and 12% for patients with and without prior chemotherapy.[50]
Is Neoadjuvant Chemotherapy Useful?
TABLE 5

Resectability Rate After Systemic Chemotherapy: Retrospective Studies
The use of new effective systemic chemotherapy has increased the possibility of obtaining a response and thus making liver metastases resectable. Several systemic chemotherapy studies have retrospectively evaluated resectability after chemotherapy (Table 5). The first retrospective reviews were written by Bismuth et al[51] and Giacchetti et al.[52] Adam and colleagues,[53] from the same group, reviewed the records of 1,104 patients with unresectable disease who received mainly FOLFOX as neoadjuvant therapy; 12.5% became resectable , and the 5-year survival rate of these patients was 34%-similar to what could be obtained with patients who were initially resectable.
TABLE 6
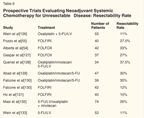
Prospective Trials Evaluating Neoadjuvant Systemic Chemotherapy for Unresectable Disease: Resectability Rate
Recently, several trials were designed to address resectability after chemotherapy (Table 6), but variations in patient selection makes it difficult to compare these studies. The Mayo Clinic trial considered patients to be unresectable if they had (1) involvement of all three major hepatic veins, portal vein bifurcation, or the hepatic vena cava; (2) involvement of the main right or main left portal vein and the main hepatic vein of the opposite lobe; (3) disease requiring more than a right or left trisegmentectomy; or (4) six or more metastatic lesions distributed diffusely in both lobes of the liver. In the presence of any of the above, patients were treated with preoperative oxaliplatin/5-FU/LV. Of 44 patients, 17 (38%) became suitable for resection, but after resection, 73% had a recurrence in the liver. Median survival was 26 months.[54] A retrospective review of these data demonstrated that 10% of patients were actually resectable prior to neoadjuvant therapy.
Pozzo et al treated 40 patients who had unresectable disease in a nonrandomized trial using neoadjuvant FOLFIRI.[55] Their criteria for unresectability were (1) six metastases or three per lobe, (2) size > 5 cm for one lesion if six metastases were present, or (3) contiguity with two hepatic veins, inferior vena cava, or liver hilum. Overall, 32% of patients were able to under-go liver resection after chemotherapy. With a median follow-up of 30 months, the disease-free survival of resected patients is presently 28 months, and overall survival has not been reached. Other trials evaluating neoadjuvant chemotherapy are listed in Table 6.
Improving Outcomes
TABLE 7

Initially Unresectable Disease: Resectability After HAI With or Without Systemic Therapy
How can we improve outcomes for unresectable patients? The use of targeted agents such as cetuximab or bevacizumab may be helpful. Rougier et al treated 23 patients with cetuximab and FOLFIRI, and 7 (30%) became resectable.[56] Would adding HAI therapy improve results? In a phase I study of oxaliplatin/irinotecan plus HAI chemotherapy in 44 clearly unresectable patients, 34% became resectable (70% of patients were receiving this treatment as second- or third-line therapy).[31] The median survival of the entire group is 36 months, and median survival for the resected group has not been reached (Table 7).
In a Japanese study of 51 patients with unresectable disease with HAI 5-FU and systemic UFT (tegafur/uracil), 31 patients were judged to be resectable after preoperative chemotherapy, but only 24 agreed to surgery. The 3- and 5-year survivals were 58% and 42% for the resected group, but for those patients who did not have resection, the 3- and 5-year survivals were 25% and 0%.[57] Using all three active agents may increase resectability. The Gruppo Oncologico Nord Ovest (GONO) randomized 244 patients to FOLFIRI or FOLFIRI plus oxaliplatin (FOLFOXIRI). In patients with only liver metastases, the resection rate was 12% and 36% for the FOLFIRI and FOLFOXIRI regimens, respectively.[58]
Predicting Poor Outcomes
Adam,[53] in a retrospective review of 131 patients who underwent liver resection after preoperative -systemic chemotherapy, found in a multivariate analysis that tumor progression on chemotherapy, elevated CA 19-9, and an increase in the number of metastases were all predictors of poor outcome.
Even if metastases disappear on CT, they may remain viable. In a review by Nordlinger et al on 586 patients[59] treated at their institution, 38 patients had disappearance of at least one lesion on CT. Pathologic examinations of sites that were considered to have a complete response showed that in 12 of 15 (80%) of these lesions, there were still viable tumor cells on pathologic examination. Areas where there was a complete response (although they could not be resected at the time of surgical exploration) were closely followed. Of these 31 sites, 23 (74%) developed a reccurrence. Clearly, this analysis shows that further postresection therapy needs to be given to these patients if sites are left intact at the time of liver resection.
Toxicity of Neoadjuvant Chemotherapy
As use of preoperative chemotherapy increases, reports are emerging about liver toxicity associated with this strategy. Investigators have found an increased incidence of steatosis, sinusoidal abnormalities, veno-occlusive disease, and steatohepatitis in this setting. In the Rubbia-Brandt[60] report on 153 patients undergoing liver resection, 51% of the 87 patients who received chemotherapy prior to resection had sinusoidal dilation, while 66 patients treated with surgery alone did not. Of 43 patients who had received prior oxaliplatin, 34 (78%) showed striking sinusoidal alterations, and 48% had veno-occlusive fibrosis.
Kooby reported on 325 patients with fatty livers undergoing resection at MSKCC. They compared this group to 160 patients with normal livers. Men and those with higher body mass index were more likely to have steatosis. Patients treated with preoperative chemotherapy were more likely to have steatosis (66%), with marked steatosis being an independent predictor of complications following hepatic resection.[61]
M. D. Anderson researchers reported no increase in preoperative deaths from neoadjuvant irinotecan in 2003.[62] However, they recently reported a 20% incidence of steatohepatitis in patients receiving preoperative irinotecan vs 4.4% in those who received no prior chemotherapy, and an 18.9% incidence of sinusoidal dilation in those receiving preoperative oxaliplatin vs 1.9% in those receiving no prior chemotherapy. Moreover, patients with steatohepatitis had an increased 90-day mortality (14.7% vs 1.6%, P = .001).[63]
Timing of Surgery and Chemotherapy
In making decisions about the timing of liver resection and pre- or postoperative chemotherapy, there are a number of factors to be considered. For patients with clearly resectable disease, resection can be done first followed by adjuvant therapy, or chemotherapy can be given prior to resection (Table 8). The advantages of preoperative chemotherapy include (1) a decrease of micrometastatic disease, (2) the ability to see if disease is responsive to a particular regimen, and (3) a decrease in tumor size. The disadvantages comprise (1) toxicity to the liver, (2) the possibility of progression, and (3) the possibility of spread to hepatic lymph nodes and a complete response make it difficult to find where to resect.
For patients with borderline resectability, chemotherapy can be done first but only for short periods of time, and as soon as response is noted, surgery should be performed rather than extending chemotherapy for a long period of time. Adam reported that prolonged chemotherapy (> 12 cycles) was related to longer hospital stays after liver resection. For clearly unresectable disease, patients should enter protocols that are addressing the question of how best to decrease liver metastases to allow resection.
In patients with synchronous colorectal cancer, there are those who advocate for simultaneous resection of both the primary and the metastatic disease. In one study of 39 consecutive patients with synchronous disease, only the histologic grade of the tumor was a predictor of survival.[64] In a retrospective series of 71 patients with five or more bilobar liver tumors, improved survival was seen with neoadjuvant therapy.[65] In a neoadjuvant study on patients with synchronous colon and liver metastases, 16 of 20 had a liver resection after chemotherapy with irinotecan/oxaliplatin/LV/5-FU. This was followed by primary resection 3 to 8 weeks later. The 2-year survival rate in these patients was 79%.[66] If the primary is in the rectum, other considerations come into play such as the need for preoperative treatment to shrink the tumor and avoid the need for a colostomy.
Ongoing Trials
Studies have begun to address the question, "Is neoadjuvant chemotherapy useful for resectable liver metastases?" The EORTC study is looking at pre- and postoperative FOLFOX vs observation. These investigators have randomized 364 patients with potentially resectable liver metastases to six cycles of FOLFOX before and after surgery, or surgery alone.[67] The primary endpoint is disease-free survival, and the study has limited accrual to patients with up to four metastases. Presently, in the group receiving preoperative chemotherapy, 7.7% progressed prior to surgery and 11% were not able to undergo resection. In the control group, 4% were not able to undergo resection. However, the actual number of patients who were resected was similar in both groups. It is too early to look at the disease-free survival and survival rates in this trial. The Southwest Oncology Group (SWOG) will conduct a study of neoadjuvant CapeOx (capecitabine/oxaliplatin) and bevacizumab.
For clearly unresectable disease, studies are asking how we can best decrease hepatic metastases to make them resectable. The North Central Cancer Treatment Group (NCCTG) and National Surgical Adjuvant Breast and Bowel Project (NSABP) are conducting a phase II trial of FOLFOX and cetuximab, Pfizer has initiated a study of FOLFIRI and bevacizumab, and MSKCC has a trial of HAI plus systemic chemotherapy with bevacizumab. Finally, Gercor is exploring FOLFOX and bevacizumab with or without erlotinib (Tarceva).
Is Adjuvant Therapy With HAI Useful Post-Liver Resection?
Once metastases grow beyond 3 mm, they obtain their blood supply from arterial circulation, whereas normal hepatocytes receive blood flow from the portal vein. After hepatic resection of liver metastases, residual disease, if present, may be 2 to 3 mm in diameter, and therefore derive its blood supply from the hepatic artery.
Four large randomized trials addressed the question of whether adjuvant therapy with HAI is useful after liver resection. At MSKCC,[68] patients were randomized after liver resection to HAI FUDR plus dexamethasone and systemic 5-FU with or without LV or systemic chemotherapy alone. The endpoint was 2-year survival. Patients were stratified according to the number of liver metastases (1, 2-4, > 4) and type of chemotherapy (none, 5-FU ± levamisole, or 5-FU + LV). Numerous other parameters, including molecular markers, were comparable in both treatment groups. The study found that the 2-year survival rate was significantly increased with HAI + systemic chemotherapy to 86%, vs 72% with systemic therapy alone (P = .03).
An update with a median follow-up time of 10 years revealed a 10-year survival rate of 41% for the HAI-plus-systemic therapy group and 27% for the systemic therapy-alone group. Overall median survivals were 68.4 vs 58.8 months for the HAI + systemic group vs systemic alone, respectively. Median time to hepatic recurrence has not been reached in the combination arm, but was 32.5 months for patients receiving systemic therapy alone (P ≤ .01). Median progression-free survival was 31 vs 17 months for HAI plus systemic therapy vs systemic therapy alone, respectively (P = .02).[69]
TABLE 9
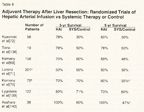
Adjuvant Therapy After Liver Resection: Randomized Trials of Hepatic Arterial Infusion vs Systemic Therapy or Control
The Eastern Cooperative Oncology Group (ECOG) and SWOG conducted a prospective trial of hepatic resection alone vs resection followed by HAI (FUDR) and systemic infusional 5-FU.[70] Only patients with three or fewer hepatic metastases were enrolled, and 109 patients were randomized prior to resection. Because randomization was done prior to resection, 29 patients were found to be ineligible at the time of liver resection. For the 75 patients who actually entered the study, 4-year disease-free survival was significantly longer with HAI plus systemic therapy vs resection alone (58% and 34%, respectively, P = .039). The primary endpoint of the study was disease-free survival, and the study was not powered for overall survival. The 5-year survival rate was 58% for the HAI-plus-systemic treatment group and 40% for the resection-only group (Table 9).
In a German multicenter, randomized trial[71] of hepatic resection alone vs hepatic resection plus adjuvant HAI 5-FU/LV, patients were stratified according to number of liver metastases (1-2, 3-6) and the site of the primary tumor (colon or upper rectum, mid or lower rectum). A total of 113 patients were assigned to each group. Despite initial randomization, only 87 (77%) were actually treated in the HAI-plus-systemic therapy group for various reasons including extrahepatic disease and technical complications. Chemotherapy data were available for 73 (64.6%) of the 113 patients randomized, and only 34 (30%) patients completed the assigned protocol, possibly due to the use of ports and 5-FU therapy. No survival advantage was seen. In the secondary analysis comparing the "actually treated" patients (n = 87) to those receiving no therapy (n = 114), median survival was 44.8 vs 39.7 months, respectively. Median time to progression in the liver doubled in the group receiving HAI 5-FU/LV (44.8 vs 23.3 months), and median time to any progression was 20 vs 12.6 months, respectively.
A prospective randomized study from Greece used intraoperative randomization to regional liver therapy plus systemic therapy vs systemic therapy alone.[139] The regional group received mitomycin, 5-FU/LV, and interleukin-2 (Proleukin) via a hepatic arterial catheter and also received the same drugs by a systemic route. The systemic group received the same drugs without HAI therapy. A total of 143 patients were randomized, 5 died during the postoperative period, and 16 were lost to follow-up, leaving 122 in the follow-up groups. The 2-year survival rates were 80% and 71%, and 5-year survival rates were 73% and 60%, in the regional-plus-systemic vs systemic-alone groups, respectively (P = .004). Five-year freedom from hepatic recurrence was also significantly increased in the regional-plus-systemic group (82% vs 49%, P < .001).
Tono et al[138] randomized 19 patients to continuous infusion of 5-FU, 500 mg/d for 4 days via HAI for 6 weeks. Though this was a small study, there was an increase in 3-year disease-free survival-66.7% for the regional group and 20% for the control group (P = .045). The 5-year survival rate was 77.8% for the regional group vs 50% for the control group.
In a nonrandomized study from Japan,[72] 58 patients who had radical resection of metastatic colorectal carcinoma could select whether they wanted HAI plus systemic therapy, or systemic therapy alone after surgery. The HAI therapy was 5-FU, 600 mg/m2/d for 2 days, with oral UFT, 200 mg twice daily for 5 days vs oral UFT alone. The 5-year survival was significantly increased for the HAI-plus-systemic therapy group (59% vs 27%, P < .001). The investigators also found a significant reduction in hepatic recurrence-7% for HAI plus systemic therapy vs 57% for systemic therapy alone (P < .001). Another small Japanese study of 38 patients involved two groups, one receiving HAI 5-FU infusion for 3 weeks and the other, no further treatment after hepatic resection. The 4-year survival rate was 100% vs 47% for the HAI and control groups, respectively (P = .05).
A meta-analysis by Clancy et al[73] suggested no increase in survival when 1- and 2-year survivals were reported. However, looking at these same studies and reporting 3- and 5-year survivals show different results (Table 9).
Newer Agents
Some phase I and phase II studies testing the new systemic chemotherapy agents with HAI as adjuvant therapy after liver resection have been completed. A phase I study at MSKCC of systemic irinotecan and HAI FUDR/dexamethasone arrived at an irinotecan dose of 200 mg/m2 every other week with concurrent HAI therapy.[74] The hepatic arterial dose (FUDR 0.12 mg × kg × 30 ÷ pump flow rate) was administered over 14 days with dexamethasone. In this adjuvant trial of systemic irinotecan and HAI FUDR/dexamethasone study, with a median follow up time of 5.2 years, the 2-year survival rate is 89% and the 5-year survival rate is 58%.
Another phase I trial used escalating systemic doses of oxaliplatin/5-FU/LV and HAI therapy.[75] With a minimum follow-up time of 26.9 months for the adjuvant systemic oxaliplatin/5-FU/LV-plus-HAI FUDR/dexamethasone trial, the 2-year survival rate is presently 98% and 5-year survival is 90%, but this may change with longer follow-up. At the Mayo Clinic, 5-FUDR plus dexamethasone has been added to systemic oxaliplatin and capecitabine. The FUDR dose is 0.2 mg/kg/d, not divided by the flow rate. Presently, the 2-year survival rate is 86%.[76]
New studies to address the usefulness of adjuvant therapy after liver resection include the National Surgical Adjuvant Breast and Bowel Project (NSABP) study of HAI plus systemic oxaliplatin plus capecitabine vs systemic oxaliplatin plus capecitabine alone. A trial at MSKCC is addressing the question of the safety of bevacizumab given with HAI plus systemic therapy after liver resection in a randomized study of HAI and systemic therapy with or without bevacizumab (Table 8).
Is Adjuvant Systemic Chemotherapy Alone Useful After Liver Resection?
Two new randomized studies have addressed the utility of systemic therapy after liver resection. In the ENG study (EORTC/NCIC CTG/GIVIO), 128 patients were randomized to receive 5-FU and LV chemotherapy for 8 weeks vs no further therapy (control) after liver or lung resection. No significant differences were seen between the two groups in terms of overall or disease-free survival (39 vs 20 months for the treated and control groups, respectively). The 4-year survival rates were 57% and 47% for the treated and control groups.[77]
A European intergroup study (FFCD) randomized 173 patients to systemic 5-FU/LV vs no further treatment after liver resection. The 5-FU and LV were given by the bolus method on a monthly schedule. The 2-year disease-free survival (endpoint) was 50.4% for those receiving chemotherapy and 38.1% for the control group (P = .058). Negative prognostic factors were synchronous disease, multiple metastases, stage III tumor, preoperative hypertension, postoperative complications, and an elevated preoperative CEA. Median survival was 62 vs 46 months for the 5-FU/LV group vs control group. The 2- and 5-year survival rates were 81% and 51% for the chemotherapy group and 82% and 41% for the control group.
A trial combining the two European studies to increase power demonstrated that there was no significant difference in progression-free survival or survival between postoperative systemic chemotherapy with 5-FU/LV vs control after curative resection.[141] Progression-free survival was 27.9 vs 18.8 months (P = .058) and overall survival was 62.2 vs 47.3 months (P = .095) for the chemotherapy vs surgery-alone groups, respectively. Patient characteristics in this study included only one metastasis in 68% of patients, and more than a 1-year disease-free interval in 57% of patients. These factors clearly can affect results, as seen in this analysis, where disease-free survival was 27 months for a single metastasis and 16.8 months for two or more metastases (P = .036) and overall survival was 64.5 months for one metastasis vs 40 months for two or more metastases. In the MSKCC study of adjuvant HAI therapy plus systemic 5-FU/LV after liver resection, 36% of patients had only one metastasis, while 19% had more than four metastases, and only 20% had a disease-free interval greater than 1 year.[68]
A small study of 29 patients addressed the efficacy of systemic irinotecan post-hepatic resection.[142] The 2-year overall survival rate in this study was 85%. The patients were in a better-risk category, with 62% having only one metastasis, 79% with a disease-free interval > 1 year, and 62% with a surgical margin > 1 cm. The median disease-free survival was 45 months. The initial dose of irinotecan (350 mg/m2) had to be lowered to 300 mg/m2, and in patients older than 70, those who had received prior radiation therapy, and those with 60% of their liver resected, it was lowered to 250 mg/m2 every 3 weeks. Current studies of systemic therapy are addressing the use of new agents alone or with molecular-targeted agents.
Cryoablation
Cryoablation is a freeze-thaw process that can induce cellular destruction. With this method, a cryoprobe is intraoperatively inserted into the lesion, liquid nitrogen is applied, and repeated freezing and thawing causes intra- and extracellular ice crystals, which destroy the tumor. One of the problems with cryoablation is that not all tumor cells may be destroyed. For example, in a study by Morris and Ross, 75% of patients treated with cryoablation developed an elevated CEA within 6 months.[78] In a large series of patients undergoing cryoablation, the actuarial 1-, 3-, and 5-year survival rates were 82%, 32%, and 13%, respectively.
Cryoablation has been used in association with liver resection in cases where all disease cannot be resected. In one trial, the 3-year survival of 75 patients who underwent resection plus cryotherapy was similar to that of 32 patients who underwent resection alone.[79] Similar results were obtained by Rivoire et al, who reported a 4-year survival rate of 36% in patients receiving cryotherapy after preoperative chemotherapy.[80] In a study examining systemic irinotecan and HAI FUDR plus dexamethasone in previously treated patients, eight patients also received cryotherapy because resection was not possible. The 2-year survival rate was 80% for the patients receiving cryotherapy with HAI plus systemic therapy.[30]
Complications of cryoablation include biliary fistulae, coagulopathy, myoglobinuria, hepatic abscesses, and pleural effusions. A survey of a number of hospitals using cryoablation found a mortality rate of 1.5%, with the most common reason for mortality being cryoshock (18%) or liver failure (12%).[81]
Radiofrequency Ablation
Heat can be used to kill tumor cells; one such method is radiofrequency ablation (RFA). High-frequency attenuating currents result in ionic agitation, which induces heat and coagulative necrosis. RFA electrodes can be guided into position by sonogram, computed tomography (CT), or magnetic resonance imaging (MRI), or can be used intraoperatively. RFA has advantages over cryoablation because of the lower cost of equipment, less time involved, and the small size of the electrodes permitting use of RFA without laparotomy. A major obstacle to its use is the size of the lesion, because as heat is generated within the tumor, charring of the tissues can decrease the conduction of heat. Patients with lesions smaller than 3 cm are good candidates.
Clinical Trials
A large multicenter Italian study of 423 patients treated with RFA reported 3- and 5-year survival rates of 47% and 24%, respectively,[82] which is similar to outcomes seen in surgical series. Similar results were obtained in another series of 25 patients, where resection was not performed because the lesion was close to a major vessel or there were medical comorbidities. The 3-year survival rate with RFA alone was 52%.[83] Elias et al used RFA instead of repeat resections for the treatment of recurrence after liver resection in 47 patients. They found that this increased the percentage of local cures and decreased the need for resection.[84]
Livraghi et al evaluated the role of RFA in 88 patients who were waiting for resection. A total of 53 patients had complete tumor ablation by RFA, and 16 (30%) remained tumor-free. Among these 53 patients with complete ablation, 98% were spared surgical resection-44% because they remained tumor-free and 56% because they developed additional metastases. Lesions in 35 (40%) of the 88 patients demonstrated local tumor recurrence. Several underwent surgical resection, whereas 15 developed unresectable disease.[85]
In a study from the Cleveland Clinic, 135 patients underwent RFA because they were not good candidates for surgical resection, and 80% of these patients had already received chemotherapy. The study was designed to look at the predictors of survival after RFA. The median overall survival was 28.9 months. Predictors of survival included size of the lesion and baseline CEA values. A median survival of 38 months was found for lesions < 3 cm, 34 months for lesions 3 to 5 cm, and 21 months for lesions > 5 cm (P = .03). The survival was also influenced by baseline CEA values, with median survivals of 34 and 16 months for CEAs < 200 ng/mL vs > 200 ng/mL (P = .01). In the Cox proportional hazards model, only the size of the largest lesion (> 5) was found to be a significant predictor of survival.[86]
In another investigation of 179 patients, size was also an important predictor. For patients with a lesion size ≤ 2.5 cm vs > 4.1 cm, the local recurrence rate was 28% and 68%, respectively.[87]
In a series of 418 patients who underwent their first laparotomy at M. D. Anderson Cancer Center for the treatment of liver metastases from colorectal cancer, 348 were treated with intent to cure: 190 had resection only, 101 had RFA and resection, and 57 had RFA alone. Recurrences were lowest with resection (52%) vs 64% for RFA and resection, and 84% for RFA alone. Liver-only recurrence after RFA was 44%. The 4-year survival rate was 65% for resection, 36% for resection and RFA, and 22% for RFA alone.[88] A multivariate analysis of these patients found that the type of procedure (ie resection, RFA and resection, or RFA alone) influenced survival, with the RFA-alone group having the lowest survival. Of course, RFA was usually a component of therapy when resection was not possible, especially in cases were the anatomic distribution of tumors made complete resection impossible. Therefore, this is not a true comparison of RFA vs resection. However, the 3-year survival rate for patients with a single metastasis treated by resection or RFA was 80% vs 40%, respectively, suggesting that RFA cannot replace resection.
The combination of RFA and HAI has shown feasibility in small studies. In an M. D. Anderson study on 50 patients, 32% remained tumor-free at a 20-month median follow-up. Recurrence at the site of RFA was seen in 10% and new liver metastases in 30%.[89] Kainuma et al treated nine patients with bilobar disease using RFA and regional chemotherapy with 5-FU, doxorubicin, and cisplatin.[90] The local recurrence rate was 55%, and the 2-year survival rate was 39%. Martin treated 21 patients with RFA and HAI FUDR. With a median follow-up time of 24 months, the median survival is 30 months.[91] No trial is presently open to address the question of HAI or HAI and systemic therapy with RFA, vs RFA alone.
Toxicity from RFA is clearly outlined in a series by de Baere et al, involving 312 patients who underwent RFA (226 of these procedures were done percutaneously). Toxicities included liver abscesses (n = 7), portal vein thrombosis (n = 3), pleural effusion (n = 5), colon perforation (n = 1), and renal insufficiency (n = 1).[92]
Role of RFA
In conclusion, presently there are no data supporting the use of RFA in resectable lesions in patients who are able to undergo resection. RFA may have a role during surgical resection when one side of the liver is resected and limited disease exists on the other side that cannot be resected but can be ablated. In patients who have undergone a resection and develop a small recurrence and/or for whom repeat surgery is not indicated, percutaneous or laparoscopic RFA can be used if the lesion can be reached easily and is not close to large vessels. The presence of blood vessels near the tumors causes conduction of thermal energy away from the tumor, and this "heat sink" in effect spares killing the tumor near the blood vessel.[93]
Whether techniques such as cryoablation and RFA are more useful than chemotherapy, or should be used in combination with chemotherapy, is not known and new trials will hopefully address this question. A European study being conducted by the EORTC is exploring the use of RFA and chemotherapy vs chemotherapy alone.
Radiation Therapy
In the past, radiation therapy has not been an effective modality for the treatment of colorectal cancer, because the liver is quite sensitive to radiation and when doses greater than 3,500 cGy are used, radiation toxicity is common. Recently, new strategies in administering radiation therapy have made such treatment more effective. These include three-dimensional (3D) conformal radiation with or without simultaneous administration of radio-sensitizing chemotherapy agents, antibody-directed radioablation, radioembolization, and interoperative interstitial radiation.
Conformal 3D treatment planning, in which beams can enter the patient from almost any angle, can substantially reduce irradiation to the normal liver.[94,95] In one study, 22 patients with localized unresectable colorectal cancer metastatic to the liver were treated with HAI FUDR (0.2 mg/kg/d) combined with up to 72.6 Gy.[96] An objective response rate of 50% was reported, with the remaining patients showing stable disease. The overall median survival was 20 months.[97] A more recent trial in 128 patients (47 with colorectal cancer) used 60 Gy of radiation with HAI FUDR. These patients had hepatic lesions too large to be treated by RFA. Approximately 60% of the colorectal cancer patients responded. The median survival was 15.8 months for the whole group and 17.2 months for the colorectal group. Grade 3 and 4 toxicity was seen in 21% and 9% of patients, consisting mostly of liver function abnormalities, upper gastrointestinal bleeding, and hematologic complications.[98]
Delivering high-dose localized radiation to part of the liver can be performed by using a single large dose of radiation.[99,100] This technique has sometimes been referred to as "stereotactic" radiation, although current methods of liver treatment lack the precision in setup and tumor localization typical of stereotactic brain irradiation. Doses of 8 to 30 Gy have been delivered in a single fraction to small tumors. In a phase I/II study by Herfarth et al, 37 patients with 60 tumors (4 primary liver tumors and a median tumor size of 10 cm), showed local tumor control in 81% at 18 months after therapy.[100]
Another method of delivering high-dose localized radiation is through the use of yttrium-90 microspheres that can be infused into the hepatic artery.[101-103] A randomized trial showed that the combination of microspheres and HAI FUDR was superior to HAI alone in the treatment of colorectal cancer confined to the liver. The overall response rate and median time to progression were increased in the group receiving microspheres (44% vs 18%, P < .01; and 16 vs 10 months, P < .001).[104] The problem with the study is the low response rate seen with FUDR alone.
A single high dose of radiation can be administered to localized hepatic metastases by employing a high-dose-rate iridium-192 afterloader placed at the time of laparotomy.[105] A relative disadvantage of this approach is that sources can be placed only at the time of a laparotomy.
Radiation can be combined with either systemic or regional chemotherapy. The fluoropyrimidines have been shown to be radiation sensitizers.[106-108] In a study comparing microspheres containing yttrium-90 plus 5-FU/LV or 5-FU/LV alone, the median survival was 29 and 13 months, respectively.[109]
Conclusions
With the introduction of more effective diagnostic and therapeutic techniques, the treatment of liver metastases has become more challenging and rewarding. We now have a much clearer view of the extent of liver metastases and the presence of extrahepatic metastases. The use of MRI, CT, and positron-emission tomography (PET) can often help distinguish benign from metastatic liver lesions. In deciding how best to treat a patient with liver metastases, a multi-disciplinarian approach is useful. Input from medical oncologists, diagnostic interventional radiologists, and hepatobiliary surgeons can ascertain whether the patient is resectable and whether resection should be done before or after chemotherapy.
In most patients who are clearly resectable, resection should be performed first. Unless studies now being conducted show a benefit to preoperative chemotherapy, it should not be done routinely unless patients are on protocol. If preoperative chemotherapy is performed in resectable patients, it should be done for short periods of time, ie, ≤ 3 months. After liver resection, adjuvant therapy should be administered. Patients should enter protocols addressing the usefulness of new drugs, molecular-targeted agents, or HAI therapy, either alone or in combination after resection. Since most recurrences occur during the first 2 years, follow-up of patients postresection should be frequent-every 3 months in the first 2 years, and then every 4 to 6 months for another 3 years.
Patients with unresectable hepatic metastases should be treated with FOLFIRI or FOLFOX with or without bevacizumab or cetuximab, in countries where it is available, or with HAI plus irinotecan and/or oxaliplatin. If patients experience major tumor reduction, subsequent resection may be possible. RFA plus chemotherapy vs chemotherapy alone is also being evaluated in unresectable patients. In patients who show disease progression on first-line therapy, second-line therapy with systemic therapy alone or with HAI may produce responses that might enable a patient to become resectable. Adjuvant therapy after liver resection is necessary. Trials have already demonstrated benefit, and further trials have been designed to determine which type of therapy will be most efficacious.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Kemeny N, Kemeny MM, Lawrence TS: Liver metastases, in Abeloff MD, Armitage JO, Niederhuber JE, et al (eds): Clinical Oncology, 3rd ed, pp 1141-1178. Philadelphia, Elsevier, 2004.
2. Tashiro T, Kawada Y, Sakurai Y, et al: Antitumor activity of a new platinum complex, oxalato (trans-l-1,2-diaminocyclohexane) platinum (II): New experimental data. Biomed Pharmacother 43:251-260, 1989.
3. Mathe G, Kidani Y, Segiguchi M, et al: Oxalato-platinum or 1-OHP, a third-generation platinum complex: An experimental and clinical appraisal and preliminary comparison with cis-platinum and carboplatinum. Biomed Pharmacother 43:237-250, 1989.
4. Saltz LB, Cox JV, Blanke C, et al: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905-914, 2000.
5. Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 355:1041-1047, 2000.
6. Goldberg RM, Sargent DJ, Morton RF, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23-30, 2004.
7. Tournigand C, Andre T, Achille E, et al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 22:229-237, 2004.
8. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
9. Rothenberg ML, Cox JV, DeVore RF, et al: A multicenter, phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal carcinoma. Cancer 85:786-795, 1999.
10. Rothenberg ML, Oza AM, Bigelow RH, et al: Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: Interim results of a phase III trial. J Clin Oncol 21:2059-2069, 2003.
11. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
12. Giantonio BJ, Catalano PJ, Meropol NJ, et al: High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the Eastern Cooperative Oncology Group (ECOG) study E3200 (abstract 2). J Clin Oncol 23(16S):1s, 2005.
13. Breedis C, Young G: The blood supply of neoplasms in the liver. Am J Pathol 30:969-977, 1954.
14. Ensminger W, Niederhuber J, Dakhil S, et al: Totally implanted drug delivery system for hepatic arterial chemotherapy. Cancer Treat Rep 65:393-400, 1981.
15. Weiss L, Grundmann E, Torhorst J, et al: Haematogenous metastatic patterns in colonic carcinoma: An analysis of 1541 necropsies. J Pathol 150:195-203, 1986.
16. Tandon RN, Bunnell IL, Cooper RG: The treatment of metastatic carcinoma of the liver by the percutaneous selective hepatic artery infusion of 5-fluorouracil. Surgery 73:118-121, 1973.
17. Lorenz M, Muller HH: Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion vs fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol 18:243-254, 2000.
18. Kemeny NE, Niedzwiecki D, Hollis DR, et al: Hepatic arterial infusion vs systemic therapy for hepatic metastases from colorectal cancer: A randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 24:1395-1403, 2006.
19. Kemeny N, Daly J, Reichman B, et al: Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 107:459-465, 1987.
20. Chang AE, Schneider PD, Sugarbaker PH, et al: A prospective randomized trial of regional vs systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg 206:685-693, 1987.
21. Hohn DC, Stagg RJ, Friedman MA, et al: A randomized trial of continuous intravenous vs hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: The Northern California Oncology Group trial. J Clin Oncol 7:1646-1654, 1989.
22. Wagman LD, Kemeny MM, Leong L, et al: A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol 8:1885-1893, 1990.
23. Martin JK, Jr., O'Connell MJ, Wieand HS, et al: Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg 125:1022-1027, 1990.
24. Rougier P, Laplanche A, Huguier M, et al: Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: Long-term results of a prospective randomized trial. J Clin Oncol 10:1112-1118, 1992.
25. Allen-Mersh TG, Earlam S, Fordy C, et al: Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet 344:1255-1260, 1994.
26. Kerr DJ, McArdle CS, Ledermann J, et al: Intrahepatic arterial vs intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: A multicentre randomised trial. Lancet 361:368-373, 2003.
27. Kemeny N, Seiter K, Niedzwiecki D, et al: A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone vs fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer 69:327-334, 1992.
28. Kemeny N, Conti JA, Cohen A, et al: Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol 12:2288-2295, 1994.
29. Kemeny N, Eid A, Stockman J, et al: Hepatic arterial infusion of floxuridine and dexamethasone plus high-dose mitomycin C for patients with unresectable hepatic metastases from colorectal carcinoma. J Surg Oncol 91:97-101, 2005.
30. Kemeny N, Gonen M, Sullivan D, et al: Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol 19:2687-2695, 2001.
31. Kemeny N, Jarnagin W, Paty P, et al: Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol 23:4888-4896, 2005.
32. Gluck WL, Akwari OE, Kelvin FM, et al: A reversible enteropathy complicating continuous hepatic artery infusion chemotherapy with 5-fluoro-2-deoxyuridine. Cancer 56:2424-2427, 1985.
33. Northover JM, Terblanche J: A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg 66:379-384, 1979.
34. Stagg RJ, Venook AP, Chase JL, et al: Alternating hepatic intra-arterial floxuridine and fluorouracil: A less toxic regimen for treatment of liver metastases from colorectal cancer. J Natl Cancer Inst 83:423-428, 1991.
35. Steele G Jr, Ravikumar TS: Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 210:127-138, 1989.
36. Hughes KS, Simon R, Songhorabodi S, et al: Resection of the liver for colorectal carcinoma metastases: A multi-institutional study of patterns of recurrence. Surgery 100:278-284, 1986.
37. Scheele J, Stangl R, Altendorf-Hofmann A: Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br J Surg 77:1241-1246, 1990.
38. Scheele J, Altendorf-Hofmann A: Resection of colorectal liver metastases. Langenbecks Arch Surg 384:313-327, 1999.
39. Ohlsson B, Stenram U, Tranberg KG: Resection of colorectal liver metastases: 25-year experience. World J Surg 22:268-277 (incl discussion), 1998.
40. Minagawa M, Makuuchi M, Torzilli G, et al: Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: Long-term results. Ann Surg 231:487-499, 2000.
41. Bolton JS, Fuhrman GM: Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg 231:743-751, 2000.
42. Makuuchi M, Thai BL, Takayasu K, et al: Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 107:521-527, 1990.
43. Makuuchi M, Hasegawa H, Yamazaki S: Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 161:346-350, 1985.
44. Vajdova K, Heinrich S, Tian Y, et al: Ischemic preconditioning and intermittent clamping improve murine hepatic microcirculation and Kupffer cell function after ischemic injury. Liver Transpl 10:520-528, 2004.
45. Launois B, Jamieson GG: The importance of Glisson's capsule and its sheaths in the intrahepatic approach to resection of the liver. Surg Gynecol Obstet 174:7-10, 1992.
46. Khatri VP, Petrelli NJ, Belghiti J: Extending the frontiers of surgical therapy for hepatic colorectal metastases: Is there a limit? J Clin Oncol 23:8490-8499, 2005.
47. Poston GJ, Adam R, Alberts S, et al: OncoSurge: A strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol 23:7125-7134, 2005.
48. Fong Y, Fortner J, Sun RL, et al: Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 230:309-321 (incl discussion), 1999.
49. Nordlinger B, Guiguet M, Vaillant JC, et al: Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 77:1254-1262, 1996.
50. Adam R, Aloia T, Figueras J, et al: LiverMetSurvey: Analysis of clinicopathologic factors associated with the efficacy of preoperative chemotherapy in 2,122 patients with colorectal liver metastases (abstract 3521). J Clin Oncol 24(18S):151s, 2006.
51. Bismuth H, Adam R, Levi F, et al: Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224:509-522 (incl discussion), 1996.
52. Giacchetti S, Itzhaki M, Gruia G, et al: Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol 10:663-669, 1999.
53. Adam R, Delvart V, Pascal G, et al: Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg 240:644-658 (incl discussion), 2004.
54. Alberts SR, Horvath WL, Sternfeld WC, et al: Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J Clin Oncol 23:9243-9249, 2005.
55. Pozzo C, Basso M, Quirino M, et al: Long-term follow-up of colorectal cancer (CRC) patients treated with neoadjuvant chemotherapy with irinotecan and fluorouracil plus folinic acid (5-FU/FA) for unresectable liver metastases (abstract 3576). J Clin Oncol 24(18S):164s, 2006.
56. Rougier P, Raoul J-L, Van Laethem J-L, et al: Cetuximab+FOLFIRI as first-line treatment for metastatic colorectal CA (abstract 3513). Proc Am Soc Clin Oncol 23:248, 2004.
57. Noda M, Yanagi H, Yoshikawa R, et al: Second-look hepatectomy after pharmacokinetic modulating chemotherapy (PMC) combination with hepatic arterial 5FU infusion and oral UFT in patients with unresectable hepatic colorectal metastases (abstract 3739). Proc Am Soc Clin Oncol 23:304, 2004.
58. Falcone A, Masi G, Brunetti I, et al: The triplet combination of irinotecan, oxaliplatin and 5FU/leucovorin (FOLFOXIRI) vs the doublet of irinotecan and 5FU/leucovorin (FOLFIRI) as first-line treatment of metastatic colorectal cancer (MCRC): Results of a randomized phase III trial by the Gruppo Oncologico Nord Ovest (G.O.N.O.) (abstract 3513). J Clin Oncol 24(18S):149s, 2006.
59. Nordlinger B, Brouquet A, Penna C, et al: Complete radiological response of colorectal liver metastases (LM) after chemotherapy: Does it mean cure? (abstract 3501). J Clin Oncol 24(18S):146s, 2006.
60. Rubbia-Brandt L, Audard V, Sartoretti P, et al: Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 15:460-466, 2004.
61. Kooby DA, Fong Y, Suriawinata A, et al: Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg 7:1034-1044, 2003.
62. Parikh AA, Gentner B, Wu TT, et al: Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg 7:1082-1088, 2003.
63. Vauthey JN, Pawlik TM, Ribero D, et al: Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065-2072, 2006.
64. Tanaka K, Shimada H, Matsuo K, et al: Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery 136:650-659, 2004.
65. Tanaka K, Adam R, Shimada H, et al: Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg 90:963-969, 2003.
66. Mentha G, Majno PE, Andres A, et al: Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 93:872-878, 2006.
67. Gruenberger T, Sorbye H, Debois M, et al: Tumor response to pre-operative chemotherapy (CT) with FOLFOX-4 for resectable colorectal cancer liver metastases (LM). Interim results of EORTC Intergroup randomized phase III study 40983 (abstract 3500). J Clin Oncol 24(18S): 146s, 2006.
68. Kemeny N, Huang Y, Cohen AM, et al: Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 341:2039-2048, 1999.
69. Kemeny NE, Gonen M: Hepatic arterial infusion after liver resection. N Engl J Med 352:734-735, 2005.
70. Kemeny MM, Adak S, Gray B, et al: Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: Surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy-an intergroup study. J Clin Oncol 20:1499-1505, 2002.
71. Lorenz M, Muller HH, Schramm H, et al: Randomized trial of surgery vs surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg 228:756-762, 1998.
72. Kusunoki M, Yanagi H, Noda M, et al: Results of pharmacokinetic modulating chemotherapy in combination with hepatic arterial 5-fluorouracil infusion and oral UFT after resection of hepatic colorectal metastases. Cancer 89:1228-1235, 2000.
73. Clancy TE, Dixon E, Perlis R, et al: Hepatic arterial infusion after curative resection of colorectal cancer metastases: A meta-analysis of prospective clinical trials. J Gastrointest Surg 9:198-206, 2005.
74. Kemeny N, Jarnagin W, Gonen M, et al: Phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol 21:3303-3309, 2003.
75. Kemeny NE, Jarnagin W, Gonen M, et al: Phase I trial of hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone (DEX) in combination with systemic oxaliplatin (OXAL), fluorouracil (FU) + leucovorin (LV) after resection of hepatic metastases from colorectal cancer (abstract 3579). J Clin Oncol 23(16S):265s, 2005.
76. Alberts SR, Mahoney MR, Donohue JH, et al: Systemic capecitabine and oxaliplatin administered with hepatic arterial infusion (HAI) of floxuridine (FUDR) following complete resection of colorectal metastases (M-CRC) confined to the liver: A North Central Cancer Treatment Group (NCCTG) phase II intergroup trial (abstract 3525). J Clin Oncol 24(18S):152s, 2006.
77. Langer B, Bleiberg H, Labianca R, et al: Fluorouracil (FU) plus l-leucovorin (l-LV) vs observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): Results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial (abstract 592). Proc Am Soc Clin Oncol 21:149a, 2002.
78. Morris DL, Ross WB: Australian experience of cryoablation of liver tumors: metastases. Surg Oncol Clin N Am 5:391-397, 1996.
79. Finlay IG, Seifert JK, Stewart GJ, et al: Resection with cryotherapy of colorectal hepatic metastases has the same survival as hepatic resection alone. Eur J Surg Oncol 26:199-202, 2000.
80. Rivoire M, De Cian F, Meeus P, et al: Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer 95:2283-2292, 2002.
81. Seifert JK, Morris DL: World survey on the complications of hepatic and prostate cryotherapy. World J Surg 23:109-114 (incl discussion), 1999.
82. Lencioni R, Crocetti L, Cioni D, et al: Percutaneous radiofrequency ablation of hepatic colorectal metastases: Technique, indications, results, and new promises. Invest Radiol 39:689-697, 2004.
83. Cheung L, van Sonnenberg E, Morrison P, et al: Radiofrequency ablation therapy. Contemporary Diagnostic Radiology 28(4):1-5, 2005.
84. Elias D, De Baere T, Smayra T, et al: Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg 89:752-756, 2002.
85. Livraghi T, Solbiati L, Meloni F, et al: Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: The "test-of-time approach." Cancer 97:3027-3035, 2003.
86. Berber E, Pelley R, Siperstein AE: Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: A prospective study. J Clin Oncol 23:1358-1364, 2005.
87. Solbiati L, Livraghi T, Goldberg SN, et al: Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 221:159-166, 2001.
88. Abdalla EK, Vauthey JN, Ellis LM, et al: Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818-827 (incl discussion), 2004.
89. Scaife CL, Curley SA, Izzo F, et al: Feasibility of adjuvant hepatic arterial infusion of chemotherapy after radiofrequency ablation with or without resection in patients with hepatic metastases from colorectal cancer. Ann Surg Oncol 10:348-354, 2003.
90. Kainuma O, Asano T, Aoyama H, et al: Combined therapy with radiofrequency thermal ablation and intra-arterial infusion chemotherapy for hepatic metastases from colorectal cancer. Hepatogastroenterology 46:1071-1077, 1999.
91. Martin RC 2nd, Scoggins CR, McMasters KM: A phase II study of radiofrequency ablation of unresectable metastatic colorectal cancer with hepatic arterial infusion pump chemotherapy. J Surg Oncol 93:387-393, 2006.
92. de Baere T, Risse O, Kuoch V, et al: Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 181:695-700, 2003.
93. Wood BJ, Ramkaransingh JR, Fojo T, et al: Percutaneous tumor ablation with radiofrequency. Cancer 94:443-451, 2002.
94. Ten Haken RK, Lawrence TS, McShan DL, et al: Technical considerations in the use of 3-D beam arrangements in the abdomen. Radiother Oncol 22:19-28, 1991.
95. Lawrence TS, Tesser RJ, ten Haken RK: An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys 19:1041-1047, 1990.
96. Robertson JM, Lawrence TS, Walker S, et al: The treatment of colorectal liver metastases with conformal radiation therapy and regional chemotherapy. Int J Radiat Oncol Biol Phys 32:445-450, 1995.
97. Mohiuddin M, Chen E, Ahmad N: Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol 14:722-728, 1996.
98. Ben-Josef E, Normolle D, Ensminger WD, et al: Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 23:8739-8747, 2005.
99. Blomgren H, Lax I, Naslund I, et al: Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 34:861-870, 1995.
100. Herfarth KK, Debus J, Lohr F, et al: Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 19:164-170, 2001.
101. Gray BN, Anderson JE, Burton MA, et al: Regression of liver metastases following treatment with yttrium-90 microspheres. Aust N Z J Surg 62:105-110, 1992.
102. Tian JH, Xu BX, Zhang JM, et al: Ultrasound-guided internal radiotherapy using yttrium-90-glass microspheres for liver malignancies. J Nucl Med 37:958-963, 1996.
103. Stubbs RS, Cannan RJ, Mitchell AW: Selective internal radiation therapy (SIRT) with 90Yttrium microspheres for extensive colorectal liver metastases. Hepatogastroenterology 48:333-337, 2001.
104. Gray B, Van Hazel G, Hope M, et al: Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 12:1711-1720, 2001.
105. Thomas DS, Nauta RJ, Rodgers JE, et al: Intraoperative high-dose rate interstitial irradiation of hepatic metastases from colorectal carcinoma. Results of a phase I-II trial. Cancer 71:1977-1981, 1993.
106. Byfield JE, Calabro-Jones P, Klisak I, et al: Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5-fluorouracil or ftorafur and x rays. Int J Radiat Oncol Biol Phys 8:1923-1933, 1982.
107. Bruso CE, Shewach DS, Lawrence TS: Fluorodeoxyuridine-induced radiosensitization and inhibition of DNA double strand break repair in human colon cancer cells. Int J Radiat Oncol Biol Phys 19:1411-1417, 1990.
108. Heimburger DK, Shewach DS, Lawrence TS: The effect of fluorodeoxyuridine on sublethal damage repair in human colon cancer cells. Int J Radiat Oncol Biol Phys 21:983-987, 1991.
109. Van Hazel G, Blackwell A, Anderson J, et al: Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy vs fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 88:78-85, 2004.
110. Kohne CH, van Cutsem E, Wils J, et al: Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 23:4856-4865, 2005.
111. Grothey A, Deschler B, Kroening H, et al: Phase III study of bolus 5-fluorouracil (5-FU)/ folinic acid (FA) (Mayo) vs weekly high-dose 24h 5-FU infusion/ FA + oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC) (abstract 512). Proc Am Soc Clin Oncol 21:129a, 2002.
112. Giacchetti S, Perpoint B, Zidani R, et al: Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18:136-147, 2000.
113. de Gramont A, Figer A, Seymour M, et al: Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938-2947, 2000.
114. Fuchs CS, Marshall JL, Mitchell EP, et al: A randomized trial of first-line irinotecan/fluoropymidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C) (abstract 3506). J Clin Oncol 24(18S):147s, 2006.
115. Saltz LB, Lenz HJ, Hochster H, et al: Randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) vs cetuximab/bevacizumab (CB) in irinotecan-refractory colorectal cancer (abstract 3508). J Clin Oncol 23(16S):248s, 2005.
116. Kemeny NE, Niedzwiecki D, Hollis DR, et al: Hepatic arterial infusion (HAI) vs systemic therapy for hepatic metastases from colorectal cancer; a CALGB randomized trial of efficacy, quality of life (QOL), cost effectiveness, and molecular markers (abstract 1010). Proc Am Soc Clin Oncol 22:252,
2003.
117. Kemeny N, Cohen A, Seiter K, et al: Randomized trial of hepatic arterial floxuridine, mitomycin, and carmustine vs floxuridine alone in previously treated patients with liver metastases from colorectal cancer. J Clin Oncol 11:330-335, 1993.
118. Neyns B, Fontaine C, Delvaux G, Di Betta D, et al: Efficacy of sequential hepatic arterial infusion of CPT-11 and MTX modulated 5-fluorouracil for patients with colorectal cancer metastatic to the liver after failure of systemic thymidilate-synthase inhibitor treatment (abstract 2301). Proc Am Soc Clin Oncol 21, 2002.
119. Cyjon A, Neuman-Levin M, Rakowsky E, et al: Liver metastases from colorectal cancer: Regional intra-arterial treatment following failure of systemic chemotherapy. Br J Cancer 85:504-508, 2001.
120. Boige V, Lacombe S, De Baere T, et al: Hepatic arterial infusion oxaliplatin combined with intravenous 5-FU and folinic acid in non resectable liver metastasis of colorectal cancer: A promising option for failures to systemic chemotherapy (abstract 1770). Proc Am Soc Clin Oncol 22:291, 2003.
121. Ducreux M, Ychou M, Laplanche A, et al: Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: A trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol 23:4881-4887, 2005.
122. de Gramont A, Cervantes A, Andre T, et al: OPTIMOX study: FOLFOX 7/LV5FU2 compared to FOLFOX 4 in patients with advanced colorectal cancer (abstract 3525). Proc Am Soc Clin Oncol 23:251, 2004.
123. Tabernero JM, Van Cutsem E, Sastre J, et al: An international phase II study of cetuximab in combination with oxaliplatin/5-fluorouracil (5-FU)/folinic acid (FA) (FOLFOX-4) in the first-line treatment of patients with metastatic colorectal cancer (CRC) expressing Epidermal Growth Factor Receptor (EGFR). Preliminary results (abstract 3512). Proc Am Soc Clin Oncol 23:248, 2004.
124. Delaunoit TP, Krook JE, Sargent DJ, et al: Chemotherapy-allowed resection of metastatic colorectal cancer: A cooperative group experience (abstract 196). Presented at the Gastrointestinal Cancers Symposium, American Society of Clinical Oncology, San Francisco, 2004.
125. Martoni A, Pinto C, Di Fabio F, et al: Phase II randomized trial on protracted 5-fluorouracil infusion plus oxaliplatin (FOX) vs capecitabine plus oxaliplatin (XELOX) as first line treatment in advanced colorectal cancer (ACRC): Preliminary results of the Italian FOCA study (abstract 3617). J Clin Oncol 23(16S):275s, 2005.
126. Wein A, Riedel C, Bruckl W, et al: Neoadjuvant treatment with weekly high-dose 5-fluorouracil as 24-hour infusion, folinic acid and oxaliplatin in patients with primary resectable liver metastases of colorectal cancer. Oncology 64:131-138, 2003.
127. Gaspar EM, Artigas V, Montserrat E, et al: Single centre study of L-OHP/5-FU/LV before liver surgery in patients with NOT optimally resectable colorectal cancer isolated liver metastases (abstract 1416). Proc Am Soc Clin Oncol 22:353, 2003.
128. Quenet F, Nordlinger B, Rivoire M, et al: Resection of previously unresectable liver metastases from colorectal cancer (LMCRC) after chemotherapy (CT) with CPT-11/L-OHP/LV5FU (Folfirinox): A prospective phase II trial (abstract 3613). Proc Am Soc Clin Oncol 23:273, 2004.
129. Abad A, Antón A, Massuti B, et al: Resectability of liver metastases (LM) in patients with advanced colorectal cancer (ACRC) after treatment with the combination of oxaliplatin (OXA), irinotecan (IRI) and 5FU. Final results of a phase II study (abstract 3618). J Clin Oncol 23:16s, 2005.
130. Falcone A, Masi G, Cupini S, et al: Surgical resection of metastases (mts) after biweekly chemotherapy with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI) in initially unresectable metastatic colorectal cancer (MCRC) (abstract 3553). Proc Am Soc Clin Oncol 23:258, 2004.
131. Ho WM, Ma B, Mok T, et al: Liver resection after irinotecan, 5-fluorouracil, and folinic acid for patients with unresectable colorectal liver metastases: A multicenter phase II study by the Cancer Therapeutic Research Group. Med Oncol 22:303-312, 2005.
132. Masi G, Cupini S, Marcucci L, et al: Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol 13:58-65, 2006.
133. Wein A, Riedel C, Kockerling F, et al: Impact of surgery on survival in palliative patients with metastatic colorectal cancer after first line treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and folinic acid. Ann Oncol 12:1721-1727, 2001.
134. Bouchahda M, Adam R, Giacchetti S, et al: Effective salvage therapy of liver-only colorectal cancer metastases with chronomodulated irinotecan-fluorouracil-oxaliplatin via hepatic artery infusion (abstract 3585). J Clin Oncol 24(18S): 167s, 2006.
135. Kemeny MM: personal communication. New York, 2005.
136. Zelek L, Bugat R, Cherqui D, et al: Multimodal therapy with intravenous biweekly leucovorin, 5-fluorouracil and irinotecan combined with hepatic arterial infusion pirarubicin in nonresectable hepatic metastases from colorectal cancer (a European Association for Research in Oncology trial). Ann Oncol 14:1537-1542, 2003.
137. Clavien PA, Selzner N, Morse M, et al: Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery 131:433-442, 2002.
138. Tono T, Hasuike Y, Ohzato H, et al: Limited but definite efficacy of prophylactic hepatic arterial infusion chemotherapy after curative resection of colorectal liver metastases: A randomized study. Cancer 88:1549-1556, 2000.
139. Lygidakis NJ, Sgourakis G, Vlachos L, et al: Metastatic liver disease of colorectal origin: The value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology 48:1685-1691, 2001.
140. Asahara T, Kikkawa M, Okajima M, et al: Studies of postoperative transarterial infusion chemotherapy for liver metastasis of colorectal carcinoma after hepatectomy. Hepatogastroenterology 45:805-811, 1998.
141. Mitry E, Fields A, Bleiberg H, et al: Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer. A meta-analysis of two randomized trials (abstract 3524). J Clin Oncol 24(18S):152s, 2006.
142. Mackay HJ, Billingsley K, Gallinger S, et al: A multicenter phase II study of "adjuvant" irinotecan following resection of colorectal hepatic metastases. Am J Clin Oncol 28:547-554, 2005.
143. Garassino I, Carnaghi C, Rimassa L, et al: Definitive results of hybrid chemotherapy with intravenous oxaliplatin and folinic acid and intra-hepatic infustion of 5-fluorouracil in patients with colorectal liver metastases (abstract 3670). J Clin Oncol 23(16S):288s,
2005.