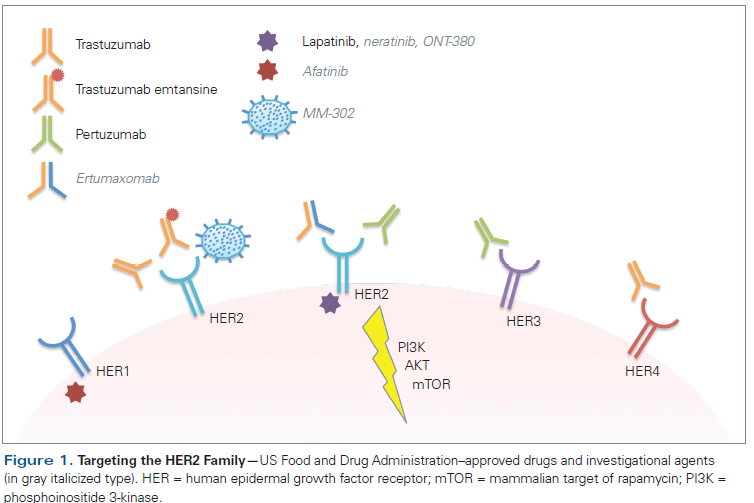
Human epidermal growth factor receptor 2 (HER2)/neu-positive breast cancer has changed from being an aggressive disease with a poor prognosis to a disease that is highly treatable, with prolonged survival possible even in patients with metastatic disease. A better understanding of HER2 biology has led to the development of powerful targeted therapies, and four drugs are already approved by the US Food and Drug Administration for treatment in the metastatic setting (trastuzumab, pertuzumab, lapatinib, and trastuzumab emtansine). Optimizing how these drugs are delivered and in what sequence is an important part of modern management of HER2-positive breast cancer. However, while the prognosis has improved, metastatic disease is still not curable; newer, better drugs are needed. This review will summarize the current standard of care; key issues that arise when treating patients with HER2-positive disease; and developments in novel therapeutics, including small-molecule inhibitors, nanoparticles, immunotherapy, and agents targeting resistance pathways.
Introduction
Invasive breast cancer is the most frequently diagnosed non- dermatologic cancer in women, with an estimated 234,190 new cases diagnosed in 2015. While disease confined to the breast and local lymph nodes is curable, this is no longer the case when it becomes metastatic, and there were approximately 40,730 breast cancer deaths in 2015.[1] Breast cancer is a heterogeneous disease; while many molecular subtypes have been identified, clinically the disease is categorized according to receptor status.[2] Approximately 20% of breast cancer tumors overexpress human epidermal growth factor receptor 2 (HER2 [ErbB2]), which is associated with a more aggressive clinical phenotype and historically portends poor prognosis.[3] However, the advent of trastuzumab, an immunoglobulin G monoclonal antibody directed against HER2, has changed the treatment paradigm of this disease.[4] There have been great advancements in HER2-targeting therapies since the development of trastuzumab, and the US Food and Drug Administration (FDA) has already approved three additional agents in the metastatic setting-pertuzumab, lapatinib, and trastuzumab emtansine (T-DM1) (Figure 1), converting HER2-positive breast cancer into a highly treatable disease, with extended survival in some patients. The complexity of disease management has likewise increased. Important questions that need to be addressed include the optimal sequence of anti-HER2 agents, how to incorporate anti-HER2 therapies in later-line settings, the appropriateness of combining anti-HER2 agents with endocrine therapy, and how to achieve lower-grade toxicity over a long-term treatment period. Furthermore, despite advances in the management of metastatic HER2-positive breast cancer, the response rate in the first-line setting ranges from 50% to 80%, and from only 20% to 40% in the second-line setting, with most patients eventually succumbing to their disease.[4-8] To improve upon the existing novel therapeutics and to develop new ones requires a better understanding of the intrinsic biology of HER2-positive disease and its interaction with extrinsic biological systems, including the immune system.
Identifying HER2
HER2 status is the most powerful predictive biomarker with which to determine the effectiveness of anti-HER2 therapy.[9] The primary means of patient assessment is through immunohistochemistry to detect HER2 protein expression on cancer cell membranes. While a score of 0 or 1 is negative and a score of 3 is positive, a score of 2 is considered equivocal and indicates the need for further testing. Fluorescent in situ hybridization (FISH) to assess HER2 gene amplification is the preferred testing method. Typically FISH is used to compare the HER2 gene copy number to the copy number of the chromosome 17 centromere (the HER2/CEP17 ratio) in a sample of the patient’s tumor tissue. Based on a joint update by the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) in 2014, a ratio of 2.0 or greater is considered positive.[10] If the score is below 2.0, the actual HER2 copy number should be assessed. A HER2 copy number of 6 or greater is considered positive, and a copy number less than 4 is considered negative. HER2 copy numbers between 4 and 6 are considered equivocal, in which case further testing should be performed.[10]
Targeting HER2 and HER3
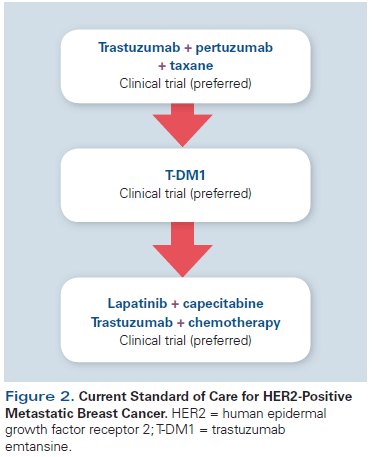
The addition of trastuzumab to chemotherapy increases response rates, progression-free survival (PFS), and overall survival (OS) in patients with HER2-positive metastatic breast cancer, and the regimen of a taxane in combination with trastuzumab was historically considered standard-of-care first-line therapy.[4] However, preclinical studies demonstrated that blockade of both HER2 and human epidermal growth factor receptor 3 (HER3 [ErbB3]) could have synergistic antitumor effects, and pertuzumab, a monoclonal antibody targeting both HER2 and HER3, was developed.[11] Early data on pertuzumab informed the design of the CLEOPATRA trial, in which patients treated with docetaxel, trastuzumab, and pertuzumab (THP) had a median PFS duration of 18.5 months; in comparison, patients randomized to placebo, docetaxel, and trastuzumab (TH), a previous standard of care for first-line therapy, had a median PFS of 12.4 months. The hazard ratio (HR) was 0.62 (95% CI, 0.51–0.75; P < .001).[6] More striking is the OS benefit, which was presented at the European Society for Medical Oncology (ESMO) 2014 Congress: The median OS with TH was 40.8 months, compared with 56.5 months in the THP arm.[7,12] Based on these data, THP is considered the current standard of care for first-line treatment of HER2-positive metastatic breast cancer (Figure 2), as per the National Comprehensive Cancer Network (NCCN) guidelines.[13] As outlined in the CLEOPATRA study design, the taxane portion of treatment should be continued for up to 8 cycles, followed by maintenance trastuzumab and pertuzumab until progression.
Trastuzumab may also be used in later-line therapies, in combination with chemotherapy. Multiple agents have been studied in combination with trastuzumab in second-line and subsequent-line settings. Although continuation of trastuzumab has not been demonstrated to improve OS prospectively, several studies have demonstrated PFS improvements with its use. As a result, and since it does not add significant additional toxicity, it is commonly employed in regimens for patients with HER2-positive disease.[14,15] Regarding pertuzumab, there are no data suggesting that continuation of pertuzumab beyond disease progression provides any benefit.
Second-Line Therapy With Antibody-Drug Conjugates
As previously noted, T-DM1 is an antibody-drug conjugate of emtansine linked to trastuzumab.[16] Emtansine is a derivative of maytansine, which was initially evaluated in phase I studies in the 1970s and found to be too toxic for systemic administration.[17] The development of a linker molecule that attaches emtansine to trastuzumab, however, provides targeted delivery of this drug to HER2-positive tumor cells. The HER2–T-DM1 complex undergoes endocytosis and degradation of the linker, ensuring maximal cytotoxic delivery to the tumor cells while limiting systemic toxicity. The EMILIA study investigated the efficacy of T-DM1 in metastatic HER2-positive breast cancer, comparing T-DM1 with capecitabine and lapatinib in patients previously treated with trastuzumab and a taxane therapy.[8] T-DM1 demonstrated a median 3.2-month PFS benefit (HR, 0.65 [95% CI, 0.55–0.77]; P < .001) and a median 5.8-month OS benefit (HR, 0.68 [95% CI, 0.55–0.85]; P < .001) compared with capecitabine plus lapatinib.[8] This study led to FDA approval of T-DM1 in the second-line setting. T-DM1 was better tolerated than lapatinib, with the most commonly reported grade 3 or 4 adverse events in the T-DM1 group including thrombocytopenia (12.9%) and elevated serum concentrations of both aspartate aminotransferase (4.3%) and alanine aminotransferase (2.9%). While the rate of serious toxicity is low, it should be noted that lower-grade toxicities, including fatigue, nausea, diarrhea, and neuropathy, may occur and can potentially affect quality of life.
Based on this initial success, T-DM1 has also been investigated in the first-line setting. A phase II study comparing T-DM1 vs TH in HER2-positive metastatic breast cancer demonstrated an improved PFS with T-DM1 (14.2 vs 9.2 months, respectively; HR, 0.59 [95% CI, 0.36–0.97]; P = .035), as well as better treatment tolerability with fewer adverse events.[18] These data provided the background for MARIANNE, a phase III randomized controlled trial comparing trastuzumab plus a taxane (docetaxel or paclitaxel) vs treatment with T-DM1 alone or T-DM1 plus pertuzumab. T-DM1 was not compared with the standard of care, THP, in either of these trials. Data from MARIANNE were presented at the 2015 ASCO Annual Meeting; it was demonstrated that treatment arms containing T-DM1 (alone or in combination with pertuzumab) in the first-line setting yielded a non-inferior PFS outcome compared with trastuzumab administered in combination with a taxane.[19] Furthermore, the addition of pertuzumab to T-DM1 did not improve PFS compared with T-DM1 alone. However, T-DM1 was associated with improved health-related quality-of-life measures and fewer high-grade adverse events. Based on these findings, it is reasonable to consider T-DM1 as an alternative in the first-line setting for select patients who are unable to tolerate taxane therapy or who have had rapid disease progression following adjuvant therapy with trastuzumab; otherwise, THP remains the preferred first-line regimen for patients with HER2-positive metastatic breast cancer, as discussed in the current NCCN guidelines.[13]
Small-Molecule Inhibitors and Toxicity
After patients have experienced disease progression on pertuzumab-based therapy and T-DM1, treatment options include trastuzumab with another chemotherapy agent as previously discussed, or with the combination of capecitabine and lapatinib.[5,15] Lapatinib is a small-molecule tyrosine kinase inhibitor (TKI) that reversibly binds to, and inhibits, the intracellular domain of HER2; it was the second anti-HER2 agent approved for management of HER2-positive metastatic breast cancer.[20] A phase III study comparing lapatinib plus capecitabine vs capecitabine alone demonstrated an improvement in time to progression (TTP) with the combination (HR, 0.49 [95% CI, 0.34–0.71]; P < .001), but an OS benefit was not demonstrated (HR, 0.92 [95% CI, 0.58–1.46]; P = .72).[5] As with many other TKIs, off-target toxicity accounted for systemic toxicity, in particular diarrhea (60% any grade) and rash (27% any grade).[5] While high-grade toxicities are less common with TKIs, lower-grade toxicities associated with their use can affect quality of life and long-term tolerability, thus limiting their clinical utility. Until the approval of T-DM1, the combination of lapatinib and capecitabine was the recommended second-line therapy; however, it is now preferable to reserve this combination for later-line treatment (Figure 2).[13]
Moving Away From Chemotherapy: Purely Targeted Approaches
Regimens optimizing survival still require chemotherapy (such as THP); however, for patients who are unable to tolerate chemotherapy or simply wish to avoid it, purely targeted regimens can be considered. As previously discussed, the MARIANNE study provides the rationale for considering T-DM1 in the first-line setting in patients who cannot be treated with standard chemotherapy. In addition, other approaches have been investigated with only targeted therapy, such as the combination of lapatinib with trastuzumab. In patients who progressed on one or more trastuzumab-containing regimens, the combination of lapatinib and trastuzumab yielded improved PFS (from 8 weeks to 11 weeks with the combination; HR, 0.74 [95% CI, 0.58–0.94]; P = .011) and OS (from 10 months to 14 months; HR, 0.74 [95% CI, 0.57–0.97]; P = .026) compared with lapatinib alone, suggesting some clinical benefit.[21] The Translational Breast Cancer Research Consortium (TBCRC) 003 study demonstrated that earlier use of these two agents in combination, such as in patients who have not received trastuzumab in the metastatic setting, has clinical activity, with an associated median PFS of 7.4 months. Nevertheless, in patients previously treated with trastuzumab, PFS decreased to 5.3 months. These studies do not show the robust PFS benefit seen with THP in the CLEOPATRA study (PFS, 18.5 months), or T-DM1 in the MARIANNE trial (PFS, 14.2 months), but combination therapy with lapatinib and trastuzumab could be considered for select patients if there are contraindications to the administration of THP or T-DM1.[6,19]
Certain patients may harbor breast cancers expressing both hormone receptors and HER2 receptors, allowing the opportunity for treatment with targeted therapies directed toward both signaling pathways. A small phase II study to investigate HER2-directed therapy and endocrine therapy in postmenopausal women with both HER2-positive and estrogen receptor (ER)-positive metastatic breast cancer found a trend toward increased TTP when trastuzumab was added to letrozole (TTP was 3.3 months with letrozole alone vs 14.1 months with letrozole plus trastuzumab), but this was not statistically significant (P = .23).[22] The larger Trastuzumab and Anastrozole Directed Against ER-Positive HER2-Positive Mammary Carcinoma (TAnDEM) study was a phase III trial evaluating anastrozole with or without trastuzumab. The investigators found that PFS was improved from 2.4 months to 4.8 months with the addition of trastuzumab (log-rank P = .0016).[23] The addition of lapatinib to letrozole has been shown to improve PFS in patients with ER-positive, HER2-positive breast cancer, from 3 months to 8.2 months.[24] While these data demonstrate that addition of trastuzumab to hormone therapy improves PFS, the results are inferior to those seen with the standard of care, THP. Notably, these studies also demonstrate the very poor PFS that can be expected in patients with ER- and HER-positive metastatic breast cancer treated with endocrine therapy alone. Nevertheless, the combination of anti-HER2 and endocrine therapy may be considered for select patients who are unable or unwilling to receive chemotherapy.
Anti-HER2 Therapy: The Next Generation
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"46022","attributes":{"alt":"","class":"media-image","id":"media_crop_7454640051120","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"5303","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","style":"height: 170px; width: 144px;","title":" ","typeof":"foaf:Image"}}]]
I. Craig Henderson, MD
Department of Medicine
Benioff Children’s Hospital
University of California, San Francisco,
San Francisco, CaliforniaWhat Are the Anti-HER2 Regimens of Choice?Patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancers commonly continue some form of anti-HER2 therapy for the remainder of their lives. Until recently, the first-line treatment of choice was trastuzumab plus a taxane (TH), but since TH plus pertuzumab (THP) has been shown to be superior to TH, it is now the preferred first-line treatment. The antibody-drug conjugate trastuzumab emtansine (T-DM1) appears to be the appropriate choice for second-line treatment, since it is superior to combination therapy with lapatinib plus capecitabine, the previous standard of care in this setting. But what should happen after that? There are insufficient data to conclude that continuation of anti-HER2 drugs after first- and second-line therapies improves clinical outcomes and, if they did, which regimens are most effective. Thus, the best choice after THP and T-DM1 is either a randomized trial directly addressing the issue of continuation or discontinuation of anti-HER2 therapy or a trial evaluating newer anti-HER2 combinations.Should Anti-HER2 Treatment Be Different for ER-Positive and ER-Negative Tumors?Triple-positive breast cancer (TPBC)-estrogen receptor(ER)+ /progesterone receptor (PR)+ /HER2+ tumors-is increasingly recognized as an entity that should be evaluated separately from other HER2-positive tumors. TPBCs are less responsive to anti-HER2 agents than ER-/HER2+ tumors, and less responsive to endocrine therapy than ER+/HER2- tumors. Use of anti-HER2 agents plus chemotherapy (eg, TH) or the addition of pertuzumab to TH (THP) are both less effective in TPBC than in ER-/HER2+ tumors. Adding an anti-HER2 agent to endocrine therapy is more effective than endocrine therapy alone in TPBCs. However, indirect comparisons suggest that administering anti-HER2 therapy plus chemotherapy is even more effective. (There are no direct comparisons.) Until we have a better understanding of TPBCs, these patients should be treated with one or two anti-HER2 agents, plus chemotherapy, plus endocrine therapy.
Despite significant advances in developing potent anti-HER2 therapies, currently approved drugs are not curative in the metastatic setting due to several mechanisms of anti-HER2 therapy resistance. Our understanding of some of the resistance mechanisms involved in HER2-positive breast cancers now guides the development of novel therapeutic strategies.
Targeting the PI3K pathway in anti-HER2 resistance
The phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is essential in HER2 signaling, and activating mutations in the pathways have been implicated in resistance to anti-HER2 drugs. Indeed, activating mutations in PI3KCA and/or loss of expression of the phosphatase and tensin homolog (PTEN) gene protein product have been identified in 20% to 50% of HER2-positive metastatic breast cancers; these correlate with poor response to trastuzumab and are associated with decreased survival.[25-28] Several studies have investigated mTOR inhibitors, such as everolimus, in patients who have progressed on trastuzumab therapy, as a mechanism of overcoming resistance to trastuzumab. A phase II study of everolimus in combination with paclitaxel and trastuzumab in patients who had already progressed on a trastuzumab-and-taxane–containing regimen found an overall response rate (ORR) of 21.8%, suggesting antitumor activity.[1] BOLERO-1, the phase III, double-blind, placebo-controlled, randomized trial counterpart in the first-line setting, did not show an improved PFS in the general cohort (HR, 0.89 [95% CI, 0.78–1.08]; P = .1166), although the ER-negative subset did appear to have an improved PFS, from 13.1 months to 20.3 months (HR, 0.66 [95% CI, 0.48–0.91]; P = .0049).[29] The phase III trial BOLERO-3, which investigated the addition of everolimus to trastuzumab and vinorelbine in trastuzumab-resistant patients, demonstrated a modest improvement in PFS from 5.78 months to 7.0 months (HR, 0.78 [95% CI, 0.65–0.95]; P = .0067); mature OS data are not yet available.[30] Investigators have also explored several important biomarkers of the PI3K and mTOR pathways, and while PI3K mutations were not predictive of response, patients with low PTEN concentrations seemed to benefit more from everolimus, suggesting that it may be a predictive biomarker for response to therapy and should be investigated further.[30] A key limitation of adding everolimus to these regimens is the significant toxicity of this drug; in the BOLERO-1 study, the everolimus group had an increased incidence of stomatitis (67% vs 32%) and diarrhea (56% vs 47%), highlighting the tradeoff of modest improvements in PFS at the expense of greater toxicity.[29]
Another novel agent of interest in this pathway is the alpha-specific PI3K inhibitor alpelisib. It is currently being studied in combination with T-DM1 in HER2-positive metastatic breast cancer, in a phase I trial that includes HER2-positive patients whose disease progressed on trastuzumab and/or a taxane-containing regimen (ClinicalTrials.gov identifier: NCT02038010). Preliminary results have demonstrated safety and significant response rates associated with alpelisib, even in patients who had previously progressed during or following treatment with T-DM1.[31]
Developments in tyrosine kinase inhibitors
To continue improving upon oral-based therapies for HER2-positive breast cancer, a new generation of irreversible HER2 TKIs is being developed (Figure 1). The majority of these second-generation TKIs have the advantage of forming covalent, irreversible bonds with the kinase domain of HER2 and are being developed with a specific focus on patients with acquired resistance to current therapies.
Afatinib, a highly selective inhibitor of epidermal growth factor receptor (EGFR; also known as HER1), HER2, and human epidermal growth factor receptor 4 (HER4) tyrosine kinase activity, has been investigated in HER2-positive breast cancer.[32] In a phase II trial in patients with HER2-positive breast cancer who had previously progressed on trastuzumab, 46% of the patients treated with afatinib achieved clinical benefit (complete response, partial response, or stable disease), and the median PFS was 15.1 weeks (95% CI, 8.1–16.7).[33] However, a high proportion of patients treated with afatinib experienced diarrhea and rash (90.2% and 65.9%, respectively), similar to the effects of other TKIs. A recently presented phase III trial comparing vinorelbine with either afatinib or trastuzumab in HER2-positive breast cancer patients who had progressed on trastuzumab-based therapy was discontinued after the experimental arm proved to be less tolerable and the OS duration was shorter compared with the other study arms.[34] Ongoing analyses of these trials are evaluating subgroups of patients who may have benefited; however, these preliminary data do not support a significant clinical benefit with afatinib.
In contrast, neratinib, an irreversible pan-HER TKI of HER1, HER2, and HER4, has demonstrated promising preclinical and clinical antitumor activity in HER2-positive breast cancer.[35,36] Phase II data in patients who had been previously treated with trastuzumab demonstrated that single-agent neratinib could produce an ORR of 24% (95% CI, 14%–36%), and in patients with no prior trastuzumab the ORR increased to 56% (95% CI, 43%–69%).[37] Notably, 93% of the patients experienced diarrhea, with 21% having grade 3 or 4. These findings served as the basis for a large phase III study (ClinicalTrials.gov identifier: NCT01808573) focused on evaluating the antitumor activity of neratinib combined with capecitabine vs the control arm of lapatinib combined with capecitabine. Essential to implementing this therapy in the clinic would be management of diarrhea, and current protocols now mandate a strict antidiarrheal regimen with loperamide administered from the outset of treatment with neratinib.
An anti-HER2 TKI that promises to have a milder adverse effect profile is ONT-380. Unlike afatinib and neratinib, ONT-380 is a reversible TKI that selectively inhibits HER2 without significant inhibition of EGFR/HER1. Currently, ONT-380 is under investigation in three phase I studies in combination with other anti-HER2 therapies (ClinicalTrials.gov identifiers: NCT01921335, NCT01983501, and NCT02025192). Results from a recently presented trial showed treatment-related grade 3 diarrhea occurred with ONT-380 doses associated with antitumor activity.[38] ONT-380 in combination with trastuzumab has also been demonstrated to have activity in the central nervous system (CNS).[39] Small molecules that can penetrate the blood-brain barrier may be of particular importance in treating CNS metastasis, which occurs in 30% to 55% of patients with HER2-positive breast cancer.[40,41]
Nanoparticles
Studies of T-DM1 demonstrated that using targeted vehicles to deliver highly toxic chemotherapy can yield significant clinical benefit.[8] In a similar approach, new therapeutic strategies are exploring the potential of packaging common toxic chemotherapies in nanoparticles conjugated with anti-HER2 antibodies. MM-302, a HER2-targeted nanoparticle containing doxorubicin, was designed to deliver doxorubicin to HER2-positive breast cancer cells (Figure 1).[42] A phase I study demonstrated that patients treated with MM-302 alone, in combination with trastuzumab, or in combination with cyclophosphamide had an ORR of 11% and median PFS of 7.6 months (95% CI, 3.6–11). Cardiac toxicity was considered acceptable, with only 9% of patients experiencing reductions in their left ventricular ejection fraction of below 50% or a greater than 10% drop from baseline.[43] These data have led to a larger phase II study, now underway, which is designed to support an application for accelerated approval of MM-302 (ClinicalTrials.gov identifier: NCT02213744).
Immune approaches
Immune evasion is a hallmark of cancers, and most anticancer agents, including trastuzumab, work best when there is an intact immune system.[44] Ertumaxomab, a bispecific antibody targeting HER2 and cluster of differentiation 3 (CD3) with selective binding to activatory Fcγ type I/III receptors, has been tested in a phase I study; it demonstrated a strong immune response and antitumor responses in 33% of patients (Figure 1).[45] A corresponding phase II study was discontinued in 2001 due to limited patient data (ClinicalTrials.gov identifier: NCT00522457). While drug development has been hampered by logistical issues, an open-label dose-escalating study with ertumaxomab in patients with HER2-expressing advanced solid tumors is ongoing (ClinicalTrials.gov identifier: NCT01569412). Other bispecific antibodies that showed promise in preclinical HER2 models are under development.[46,47]
Immune checkpoint blockade has historically been found to elicit responses in melanoma and lung cancers; however, recent phase I data in metastatic triple-negative breast cancer show response rates ranging from 18.5% to 24% using inhibitors of programmed death 1 (PD-1) and its ligand (PD-L1).[36,48,49] Given the importance of tumor-infiltrating lymphocytes, particularly in HER2-positive cancer, immune checkpoint inhibition may be an important therapeutic strategy.[50] The International Breast Cancer Study Group (IBCSG) is conducting a phase II investigation of trastuzumab combined with the anti–PD-1 inhibitor pembrolizumab (ClinicalTrials.gov identifier: NCT02129556).
Approach to Brain Metastasis
Approximately 10% to 16% of patients with metastatic breast cancer will develop brain metastasis, and among this group the predominant subtype is HER2-positive disease.[51,52] Although current ASCO recommendations do not support the use of routine screening MRIs for patients with metastatic breast cancer, a lower threshold should be considered in patients with HER2-positive disease because of the increased incidence of brain metastasis.[53] Approach to treatment of brain metastasis is a multidisciplinary effort involving neurosurgeons, as well as medical and radiation oncologists. Primary modalities of therapy include surgery, stereotactic radiosurgery, whole-brain radiation therapy, and systemic treatments that can penetrate the blood-brain barrier.[53] Systemic options for patients with HER2-positive breast cancer include capecitabine and lapatinib, since both agents can cross the blood-brain barrier. Response rates range from 0% to 67% (with an average response rate of 22.7%), and TTP/PFS ranges from 2.4 to 5.5 months (with an average of 3.9 months) with this regimen.[54] Given limited treatment options, clinical trials should be considered when appropriate, since many agents are being investigated in this setting.[53]
Conclusions
Targeting HER2 in breast cancer has led to improved outcomes for patients with metastatic breast cancer, and resulted in FDA approval of four anti-HER2 agents. While these drugs improve survival, they are generally not curative. Continued research into the biology of HER2-positive breast cancer, including investigation of resistance pathways and their interaction with our immune system, is crucial for advancing to the next generation of therapies. Therefore, modern oncology care should include clinical trials, and patients should be encouraged to participate in these when they start a new line of therapy (Figure 2).
Financial Disclosure:Dr. Jain has received research funding from Novartis. The other authors have no significant financial relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Hurvitz SA, Dalenc F, Campone M, et al. A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy. Breast Cancer Res Treat. 2013;141:437-46.
2. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-52.
3. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-82.
4. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-92.
5. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-43.
6. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109-19.
7. Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461-71.
8. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783-91.
9. Fornier M, Risio M, Van Poznak C, Seidman A. HER2 testing and correlation with efficacy of trastuzumab therapy. Oncology (Williston Park). 2002;16:1340-58.
10. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241-56.
11. Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343-6.
12. Swain S, Kim S, Cortes J, et al. Final overall survival (OS) analysis from the CLEOPATRA study of first-line (1L) pertuzumab (Ptz), trastuzumab (T), and docetaxel (D) in patients (pts) with HER2-positive metastatic breast cancer (MBC). Ann Oncol. 2014;25(suppl 4):abstr 350O_PR.
13. National Comprehensive Cancer Network. NCCN Guidelines Version 1.2016: Breast Cancer [1/14/2016]. http://www.nccn.org. Accessed January 14, 2016.
14. Manguso N, Gangi A, Giuliano A. Neoadjuvant chemotherapy and surgical management of the axilla in breast cancer: a review of current data. Oncology (Williston Park). 2015;29:733-8.
15. von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol. 2009;27:1999-2006.
16. Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280-90.
17. Chabner BA, Levine AS, Johnson BL, Young RC. Initial clinical trials of maytansine, an antitumor plant alkaloid. Cancer Treat Rep. 1978;62:429-33.
18. Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157-63.
19. Ellis PA, Barrios CH, Eiermann W, et al. Phase III, randomized study of trastuzumab emtansine (T-DM1) +/− pertuzumab (P) vs trastuzumab +/− taxane (HT) for first-line treatment of HER2-positive MBC: primary results from the MARIANNE study. J Clin Oncol. 2015;33(suppl):abstr 507.
20. Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85-94.
21. Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 study. J Clin Oncol. 2012;30:2585-92.
22. Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - results of the eLEcTRA trial. Breast. 2012;21:27-33.
23. Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2–positive, hormone receptor–positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529-37.
24. Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2009;27:5538-46.
25. Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647-56.
26. Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117-27.
27. Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395-402.
28. Chandarlapaty S, Sakr RA, Giri D, et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18:6784-91.
29. Hurvitz SA, Andre F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816-29.
30. Andre F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580-91.
31. Jain S, Nye L, Santa-Maria C. Phase I study of alpelisib and T-DM1 in trastuzumab-refractory HER2-positive metastatic breast cancer. Presented at the 38th Annual CTRC-AACR San Antonio Breast Cancer Symposium; December 8-12, 2015; San Antonio, TX. Poster P6-13-11.
32. Geuna E, Montemurro F, Aglietta M, Valabrega G. Potential of afatinib in the treatment of patients with HER2-positive breast cancer. Breast Cancer (Dove Med Press). 2012;4:131-7.
33. Lin NU, Winer EP, Wheatley D, et al. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res Treat. 2012;133:1057-65.
34. Harbeck N, Huang CS, Hurvitz S, et al. Randomized phase III trial of afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one prior trastuzumab treatment: LUX-Breast 1. Cancer Res. 2015;75(9 suppl):abstr P5-19-01.
35. Canonici A, Gijsen M, Mullooly M, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4:1592-605.
36. Ben-Baruch NE, Bose R, Kavuri SM, et al. HER2-mutated breast cancer responds to treatment with single-agent neratinib, a second-generation HER2/EGFR tyrosine kinase inhibitor. J Natl Compr Canc Netw. 2015;13:1061-4.
37. Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301-7.
38. Hamilton EP, Yardey DA, Hortobagyi GN, et al. Phase 1b study of ONT-380, an oral HER2-specific inhibitor, in combination with capecitabine (C) and trastuzumab (T) in third-line + treatment of HER2+ metastatic breast cancer. J Clin Oncol. 2015;33(suppl):abstr 602.
39. Ferrario C, Welch S, Chaves JM, et al. ONT-380 in the treatment of HER2+ breast cancer central nervous system (CNS) metastases (mets). J Clin Oncol. 2015; 33(suppl):abstr 612.
40. Lin NU, Amiri-Kordestani L, Palmieri D, et al. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19:6404-18.
41. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834-43.
42. Geretti E, Leonard SC, Dumont N, et al. Cyclophosphamide-mediated tumor priming for enhanced delivery and antitumor activity of HER2-targeted liposomal doxorubicin (MM-302). Mol Cancer Ther. 2015;14:2060-71.
43. LoRusso P, Krop I, Miller K, et al. A phase I study of MM-302, a HER2-targeted PEGylated liposomal doxorubicin, in patients with HER2+ metastatic breast cancer. Cancer Res. 2015;75(15 suppl):abstr CT234.
44. Perez EA, Thompson EA, Ballman KV, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group N9831 adjuvant trastuzumab trial. J Clin Oncol. 2015;33:701-8.
45. Kiewe P, Hasmuller S, Kahlert S, et al. Phase I trial of the trifunctional anti-HER2 × anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12:3085-91.
46. Junttila TT, Li J, Johnston J, et al. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res. 2014;74:5561-71.
47. Turini M, Chames P, Bruhns P, et al. A FcγRIII-engaging bispecific antibody expands the range of HER2-expressing breast tumors eligible to antibody therapy. Oncotarget. 2014;5:5304-19.
48. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
49. Emens LA, Braiteh FS, Cassier P, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res. 2015;75(suppl):abstr 2859.
50. Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1:448-54.
51. Arvold ND, Oh KS, Niemierko A, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136:153-60.
52. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349-54.
53. Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2100-8.
54. Lin NU. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 2013;7:307.