Managing Early-Stage Breast Cancer in Your Older Patients
As the aging population in the United States continues to grow, the incidence of diseases of the elderly, such as breast cancer, are increasing. Many more elderly women are expected to be diagnosed with new breast cancers, most of them in an early stage. Appropriate treatment of these women is important, as they have poorer outcomes when undertreated. In this review, we will discuss the biology and treatment of early breast cancer in elderly women. We will focus on the role of comorbidity and its effect on life expectancy, treatment decisions, current recommendations for primary treatment with surgery, radiation and neoadjuvant strategies, and adjuvant treatment including local radiation therapy and systemic treatment with endocrine therapy, chemotherapy, and newer agents. Finally we will discuss the importance of clinical trials in the elderly.
As the aging population in the United States continues to grow, the incidence of diseases of the elderly, such as breast cancer, are increasing. Many more elderly women are expected to be diagnosed with new breast cancers, most of them in an early stage. Appropriate treatment of these women is important, as they have poorer outcomes when undertreated. In this review, we will discuss the biology and treatment of early breast cancer in elderly women. We will focus on the role of comorbidity and its effect on life expectancy, treatment decisions, current recommendations for primary treatment with surgery, radiation and neoadjuvant strategies, and adjuvant treatment including local radiation therapy and systemic treatment with endocrine therapy, chemotherapy, and newer agents. Finally we will discuss the importance of clinical trials in the elderly.
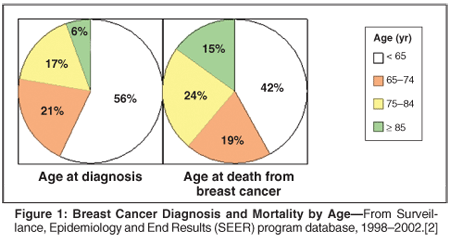
Mortality from breast cancer is decreasing, yet breast cancer incidence is rising in the United States and, as the population grows and ages, so does the absolute number of new breast cancers diagnosed.[1] The increase in new breast cancers will be particularly dramatic in the elderly, as increasing age remains the greatest risk factor for developing the disease.[1] Breast cancer is the most common cancer diagnosis in US women, with a current median age of 61 years old at diagnosis.[2] Moreover, most women who die of breast cancer are over the age of 65 (Figure 1). It is estimated that by 2030, 20% of Americans will be 65 years and older. If cancer incidence rates continue to rise, it is estimated that absolute numbers of new breast cancer cases will double by 2050, the majority being in older women.[3] It is therefore becoming increasingly important to understand how to treat elderly women with early breast cancer.
Stage at Presentation and Tumor Biology
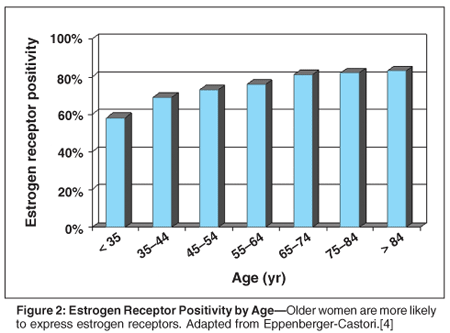
The biologic characteristics of breast cancer in older women are different from those in younger women, but whether these differences result in tumors with a more indolent prognosis is controversial. Older women are more likely to express estrogen (ER) and progesterone receptors (PR), which improves their prognosis by making them candidates for adjuvant endocrine therapy (Figure 2).[4] As women age, their breast cancers are associated with a decreased expression of markers of tumor growth and aggressiveness, including lower tumor grade, a lower S-phase fraction, more frequent diploidy, normal p53 levels, lack of HER2 (cerbB2) and epidermal growth factor receptor expression, and a lower probability of being node-positive.[5] Regardless of age, infiltrating ductal carcinoma is the most common pathologic subtype.
Diab and colleagues reviewed tumor biology and outcomes from the Surveillance, Epidemiology and End Results (SEER) database in women over 65 years old and found that despite markedly decreased rates of surgery, radiotherapy (RT), and chemotherapy, older women with breast cancer had a good prognosis.[5] Women over the age of 70 with node-negative tumors had an 8-year overall survival equivalent to that of the non-breast cancer age-matched population; women with lymph node-positive tumors had only a modest decrease in overall survival. The authors suggest that older women have more indolent disease and require less screening and treatment, but these conclusions are controversial.[6,7]
Singh et al reviewed outcomes of women with early breast cancer treated with mastectomy alone from 1927 to 1987 at a single institution and found that breast cancer was not more indolent in the 251 women who were over 70 years old.[8] Compared to younger women, the older women were less likely to have lymph node-positive tumors. However, when stage was accounted for, mortality rates were similar across age groups. Women over 70 with node-negative disease had lower distant disease-free survival than patients from 40 to 70 years old (65% vs 81% at 10 years, P = .018). Patients with node-positive disease, however, did not have a significantly different overall survival at 10 years (33% vs 38%).
Geriatric Assessment
In treating older women with breast cancer, it is important to account for the increased effect that comorbidity, limitations in functional status, and decreased life expectancy have in balancing the risks and benefits of both primary and adjuvant treatment.
Comorbidity and Mortality
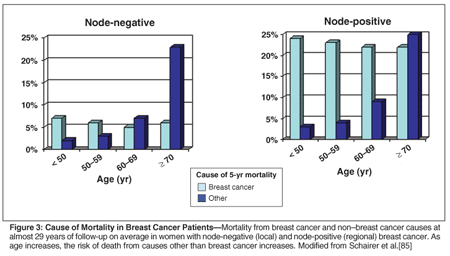
The presence of other, coexisting medical conditions can affect a woman's ability to tolerate specific treatments and decreases the non-breast cancer survival rate, regardless of age. As age increases, the risk of death from causes other than breast cancer increases (Figure 3). Satariano and Ragland noted a significant decrease in 3-year overall survival and an increase in non-breast cancer mortality in women with multiple comorbid conditions.[9] Yancik et al found that six comorbidities-diabetes, renal failure, stroke, prior malignancy, liver disease, and smoking-predicted increased mortality in postmenopausal women with breast cancer.[10]
Carey et al have created a useful prognostic index for 2-year mortality in community dwelling elders over 70 years old. Using a point scale of six items, age, gender, self-report of one activity of daily living, one instrumental activity of daily living, and two measures of physical functioning patients could reliably be divided into low-, intermediate-, and high-risk groups with a 3%-5%, 11%-12%, and 34%-36% 2-year mortality, respectively.[11]
Functional Status
Functional status impacts survival independently of age and comorbidity, and poor performance status (as measured by tools such as the Karnofsky and Eastern Cooperative Oncology Group [ECOG] performance scales) correlates with worse outcomes in cancer patients.[12]
Comprehensive Geriatric Assessment
The National Comprehensive Cancer Network and the International Society of Geriatric Oncology (SIOG) recommend the use of a comprehensive geriatric assessment (CGA) when planning treatment in the elderly.[13] The CGA is a structured evaluation of multiple domains, including physical and functional status (activities of daily living, instrumental activities of daily living, and performance status), comorbidity, socioeconomic issues, polypharmacy, nutritional status, and geriatric syndromes (delirium, dementia, depression, incontinence, falls, spontaneous bone fractures, failure to thrive, neglect, and abuse).
The CGA has been tested in oncologic practice and has been found to detect problems that directly affect cancer treatment.[14] The primary barrier to the routine use of CGA in oncology practice is time, but a short CGA, tailored for use in the outpatient oncology setting, is being tested.[12,15] Regardless of whether a formal CGA is used, when evaluating the elderly patient particular attention should be paid to cognition (memory and orientation), comorbidity (psychiatric, neuropsychiatric, and medical), polypharmacy, social issues (living conditions, caregivers, and transportation), dependence in activities of daily living, and the presence of geriatric syndromes.[15]
Primary Treatment of Early-Stage Breast Cancer in the Elderly
Surgical Treatment
The standard primary therapy for invasive breast cancer is surgical resection, with either mastectomy or breast-conserving therapy. Either of these procedures may be appropriate for the vast majority of breast cancer patients and are well tolerated regardless of age.[16] In a series of women 70 years old and older treated with modified radical mastectomy, Singletary et al found a perioperative mortality rate of 1.6% and a wound complication rate of 7.6%-a complication rate similar to that seen in younger women.[17] Older women who were not treated with appropriate surgery had worse outcomes. In some patients with significant comorbidity, dependence in activities of daily living, or geriatric syndromes, surgical treatment may be of greater risk than benefit; some of these women may be best treated with endocrine therapy if they have hormone receptor-positive tumors.
Most women, including elderly women, prefer breast-conserving therapy (partial mastectomy, sentinel node or axillary dissection, and breast RT) to modified radical mastectomy with axillary assessment. Several randomized trials with 20 years of follow-up confirm the equivalence of these treatments.[18-21] Age is not a contraindication to breast-conserving therapy, and good outcomes have been reported in women over 70 years old.[21] Although quality of life in older women is similar after both procedures, breast-conserving therapy is associated with lower rates of arm problems.[22]
Despite the benefits and preference for breast-conserving surgery, many studies find that older women have significantly lower rates of such procedures.[16,23,24] If there are no surgical contraindications to breast-conserving surgery, older women should be offered the option.
• Axillary Evaluation-Current SIOG guidelines recommend the axilla be treated similarly, regardless of age, with either sentinel lymph node dissection (SLND) or axillary lymph node dissection (ALND).[25] The utility of ALND as a treatment for older women has been questioned. Mandelblatt et al found that women over age 66 treated with ALND had worse quality-of-life scores, primarily due to arm problems and difficulties with function.[26] Martelli et al retrospectively reviewed outcomes in 761 women 70 years old and older treated with breast-conserving surgery and adjuvant tamoxifen for early breast cancer with clinically negative lymph nodes. They found that ALND decreased axillary recurrence at 10 years (5.9% vs 0%), but had no effect on the incidence of recurrence or breast cancer mortality.[27]
Knowing whether a breast cancer is node-positive or negative may significantly change prognosis and recommendations for adjuvant therapy. Nodal status, however, can also be evaluated with SLND. SLND has been shown to be safe, with few side effects in elderly women. However, for women in whom the results of axillary evaluation would not affect treatment, it is acceptable to forgo lymph node dissection.[28]
Neoadjuvant Systemic Therapy
Neoadjuvant therapy with either chemotherapy or endocrine therapy is used to reduce the size of the primary tumor and allow mastectomies in previously inoperable lesions or breast-conserving surgery in lesions formerly amenable only to mastectomy. Endocrine agents effective in the neoadjuvant setting include tamoxifen and aromatase inhibitors (such as anastrozole [Arimidex] or letrozole [Femara]) in patients who are hormone receptor-positive; chemotherapy regimens can be used in all patients regardless of hormonal status.[29,30] Though fewer data exist for endocrine therapy in this setting, clinical response rates are similar to those seen for chemotherapy, and endocrine therapy may be preferred by many older patients.
Radiation Therapy
Radiation therapy decreases ipsilateral breast cancer recurrence after breast-conserving surgery and chest wall recurrence after mastectomy. Such treatment is well tolerated by elderly women.[31]
Radiation after breast-conserving surgery provided a small mortality benefit in a meta-analysis of 15 trials (hazard ratio [HR] without radiation = 1.086, confidence interval [CI] = 1.003-1.175).[32] Most of the trials had an upper age cutoff, limiting subgroup analysis of older patients. Later trials have questioned the utility of RT in this population, particularly in older women with small tumors. In a large randomized trial of breast-conserving surgery with or without breast radiation, women over 55 years old who did not receive RT had fewer axillary recurrences than younger women without RT (3.8% vs 8.8%).
Node-negative women with hormone receptor-positive tumors treated with breast-conserving surgery and tamoxifen have been randomized to RT or no RT in two studies. Fyles and colleagues randomized 796 women over 50 and found that although RT reduced local recurrence risk from 7.7% to 0.6% (P < .001) and axillary recurrence from 2.5% to 0.5% (P = .049), no significant difference was found in overall survival.[33] The locoregional benefit of RT was decreased in women over 60 years old and in women with small tumors (< 1 cm).
Hughes et al randomized 636 women 70 years old and older, with hormone receptor-positive tumors less than 2 cm, and found that RT decreased locoregional recurrence from 4% to 1% (P < .001) without affecting distant metastases or overall survival.[34] These results were different from those of a nonrandomized population study from Canada. In that study, women over 70 had higher rates of locoregional recurrence than their younger counterparts with node-positive disease or large tumors (> 5 cm) and equal rates with node-negative disease or smaller tumors.[35]
Given the conflicting results and the lack of survival benefit, it is reasonable to offer older women with small, node-negative tumors the option of omitting RT, despite the modestly increased risk of axillary recurrence. We suggest, however, that breast irradiation be recommended to older women with a life expectancy greater than 5 years, particularly those with large tumors, positive lymph nodes, or negative hormone receptors. Further clinical trials are needed to help clarify these issues and to assess the utility of newer schedules and methods of radiation, such as hypofractionated and intensity-modulated RT.
Endocrine Therapy as Alternative Primary Treatment
Surgery is the first-line therapy for early breast cancer. However, some women are not operable candidates due to comorbid conditions, dependent activities of daily living, or severe geriatric syndrome. For these women and for patients who choose not to have surgery, tamoxifen or an aromatase inhibitor is an alternative treatment option if they are hormone receptor-positive. Akhtar et al treated 100 frail, elderly women with tamoxifen alone and achieved a 40% clinical complete response rate, a 28% partial response rate, and a 22% rate of disease stabilization, with duration of response lasting several years in some.[36]
In randomized trials, older patients randomized to surgery vs tamoxifen had similar overall survival (median survival = 6.8 vs 7.0 years, P = .001) but worse rates of locoregional control (progression-free survival = 2.3 vs 5.2 years, P = .0006).[37-39] Shortened overall survival was associated with increasing age (HR = 1.3, P = .024) and worse performance status (HR = 1.6, P = .029 for performance status > 0). Not surprisingly, other randomized trials of tamoxifen alone vs surgery followed by adjuvant tamoxifen in elderly women have also found increased locoregional recurrences with tamoxifen alone.[40,41] More recently, aromatase inhibitors have been shown to be as effective as and possibly superior to tamoxifen as neoadjuvant therapy in hormone receptor-positive postmenopausal women.[42]
Systemic Adjuvant Treatment in Elderly Women
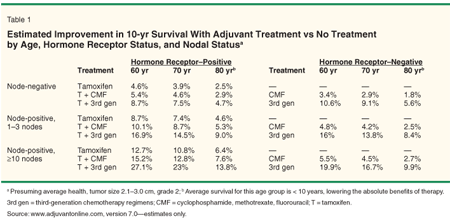
In weighing the benefits and risks of adjuvant therapy, three factors must be accounted for: breast cancer-specific prognosis, non-breast cancer prognosis, and treatment-related toxicity. Breast cancer-specific prognosis depends on factors such as tumor size, nodal status, tumor grade, hormone receptor status, and HER2 overexpression. Non-breast cancer-specific prognosis depends on life expectancy, which is related to age and comorbidity. Excellent tools exist to synthesize these factors, including the Web-based Adjuvant! Online model (www.adjuvantonline.com)[43] (Table 1) and published tables, such as those created by Extermann and others.[44]
EBCTCG and Other Trials
The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) has assessed 15 years of follow-up data in their meta-analysis of early trials of adjuvant therapy.[45] The size of the database allows analysis of small but vital treatment-related differences in different subgroups, including the elderly. The database does not include newer therapies, such as aromatase inhibitors and novel chemotherapy regimens.
Hormone Receptor-Positive Tumors
Endocrine agents such as tamoxifen and the aromatase inhibitors reduce disease recurrence and mortality in hormone receptor-positive tumors only. These agents are well tolerated, having minimal side effects relative to benefits (Table 2). For women with tumors smaller than 1 cm and/or major comorbidity, the benefit of endocrine therapy may be minimal.
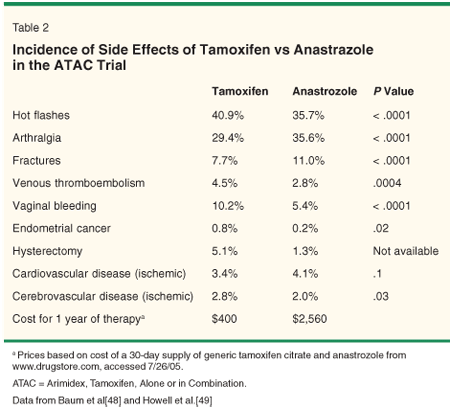
• Tamoxifen-In the EBCTCG trials, a 5-year course of tamoxifen decreased annual breast cancer mortality by 31% in hormone receptor-positive postmenopausal women regardless of age, an effect that "carries over" for at least 10 more years of risk reduction.[45,46] Side effects, including vasomotor symptoms (hot flashes), endometrial carcinoma, and thomboembolic disease, were infrequent and did not increase the likelihood of dying of non-breast cancer-related causes.
• Aromatase Inhibitors-In direct comparisons with tamoxifen, aromatase inhibitors improve disease-free survival by about 3% to 5%. To date, overall survival has not been improved except in the MA.17 trial, where the addition of letrozole after 5 years of tamoxifen showed a survival benefit compared to placebo in node-positive women (HR = 0.61, CI = 0.38-0.98, P = .04).[47]
In the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, postmenopausal women received 5 years of anastrozole, tamoxifen, or both.[48,49] Anastrozole alone was found to improve progression-free survival compared to tamoxifen or the combination, and the effect of anastrozole was most pronounced in women over 64 years old (HR = 1.19, CI = 1.04-1.36). The Intergroup Exemestane Study (IES) randomized women who had taken 2 to 3 years of tamoxifen to either the continuation of tamoxifen or a switch to exemestane (Aromasin) for the remainder of 5 years.[50] Switching to an aromatase inhibitor improved disease-free survival (86.8% vs 91.5%, CI = 85.1%-88.3% and 90.00%-92.7%). Recurrence rates also decreased in a trial of a similar design, where patients were switched from tamoxifen to anastrozole after 2 to 3 years (HR = 0.60, CI = 0.44-0.81, P = .0009).[51]
American Society of Clinical Oncology (ASCO) guidelines recommend using aromatase inhibitors in postmenopausal women with hormone receptor-positive tumors at some point in the adjuvant setting, but they note that there was insufficient evidence as to which aromatase inhibitor to select and when to use it. As aromatase inhibitors are strongly associated with increased osteoporotic fractures, the ASCO guidelines recommend baseline and yearly bone densitometry during treatment with an aromatase inhibitor.[52] These agents represent a major option for adjuvant endocrine therapy in older patients.
Adjuvant Chemotherapy
Adjuvant chemotherapy provides a smaller benefit, has more side effects, and is more complicated to administer than endocrine therapy. The greatest benefit of adjuvant therapy is seen in women with the highest risk for recurrence, the fewest comorbidities, and the longest life expectancy. The benefit is smaller in women with low recurrence risk and in women with hormone receptor-positive tumors, since use of endocrine therapy improves prognosis and thus decreases the absolute benefit of chemotherapy. The same tools used to estimate the benefit of adjuvant endocrine therapy in older women also provide guidance in the use of chemotherapy (Table 1).[43,44]
• EBCTCG Overview-After 15 years of average follow-up in the overview meta-analysis, the benefit of adjuvant chemotherapy persists but decreases with age. A trend to benefit was seen in women over 70 years old, but with only about 1,200 women over 70 enrolled in chemotherapy trials, the overview is underpowered to conclude that these benefits are significant. Regardless of age, anthracycline containing regimens were modestly better than CMF (cyclophosphamide, methotrexate, fluorouracil [5-FU]).[45] More aggressive chemotherapy such as taxane-containing and dose-dense regimens were not evaluated in the overview.
Node-Negative Tumors
Few elderly women with node-negative tumors will benefit from adjuvant chemotherapy. In a SEER database analysis, the 8-year overall survival for women over 70 with node-negative early breast cancer was no different from that of women without breast cancer.[5] In women over 60 years old with stage I disease, Desch and colleagues used a Markov model and found that chemotherapy decreased recurrence risk slightly and improved active life expectancy by only 2 weeks.[53]
• IBCSG-The International Breast Cancer Study Group (IBCSG) trial VII evaluated the effect of tamoxifen with or without three cycles of CMF in women with node-negative disease. Women who were 60 years old and older with ER-negative tumors had improved overall survival (81%-89%), but no benefit was seen in women with ER-positive tumors, small tumors (< 1 cm), or tumors with a low histologic grade.[54]
• NSABP-The National Surgical Adjuvant Breast and Bowel Project (NSABP) analyzed the effect of age on adjuvant chemotherapy for node-negative women in several trials, including NSABP B-13, NSABP B-14, NSABP B-19, NSABP B-20, and NSABP B-28.[55,56] In hormone receptor-positive women, a trial of MF (methotrexate and 5-FU) vs no chemotherapy and CMF vs no chemotherapy found improved disease-free survival and overall survival with both MF and CMF, but the effects decreased with age. In hormone receptor-negative women the benefit of adding CMF to tamoxifen decreased with age and disappeared in women over 59 years old.
Node-Positive Tumors
• EBCTCG Overview-In the overview, chemotherapy has a greater effect in women with node-positive early breast cancer than in women with node-negative early breast cancer, regardless of age. In ER-positive women with a 50% mortality risk, tamoxifen decreased the risk to 38%, and adding anthracycline-based chemotherapy to tamoxifen reduced it to 31.8%.[45] Large trials of current chemotherapy regimens still have poor accrual of elderly patients, limiting their generalizability. Studies specific to older women show that less-intense chemotherapy regimens provide more benefit compared to no treatment. However, a recent meta-analysis shows that, even in older patients, more-intense regimens provide greater benefit than less-intense regimens.[57,58]
• FASOG 08 Trial-The French Adjuvant Study Group (FASOG) 08 trial randomized 338 women over 65 years old with node-positive early breast cancer to tamoxifen with or without six cycles of weekly low-dose epirubicin (Ellence). In a univariate analysis, epirubicin showed a trend to improved disease-free survival (69.3 vs 72.6%, P = .14), which became significant in multivariate analysis (HR = 1.93 (CI = 1.7-2.17).[57] No survival benefit was noted.
• CALGB Trials-In a Cancer and Leukemia Group B (CALGB) meta-analysis of four trials of chemotherapy in node-positive early breast cancer, disease-free and overall survival were prolonged by higher-dose/intensity chemotherapy regimens, and the overall benefit persisted in a subgroup analysis of the 8% of women who were 65 years or older.[58] The older women had worse overall survival, explained by increased non-breast cancer-related mortality. Side effects, however, were greater, as was treatment-related mortality, which increased with age-from 0.2% in women 50 years and under to 1.5% in women 65 years and older (P < .001).
Chemotherapy Toxicity
In many studies, older women receiving chemotherapy exhibit more toxicity than their younger counterparts.[59] Arguments have been made that older patients should receive modified regimens. However, while patients may have fewer side effects when given less-intensive chemotherapy regimens, the benefits of treatment may be smaller. A brief review of several issues pertinent to chemotherapy in the elderly is outlined below. For more detail, several good reviews of chemotherapy in the elderly and of toxicity of adjuvant chemotherapy for early breast cancer are available.[59-61]
• Renal Function-Glomerular filtration rate decreases with age and should be considered when giving renally cleared medications to the elderly. An interesting study has shown that CMF given for metastatic breast cancer, with dose adjustment for creatinine clearance, improved toxicity without affecting response.[62]
• Hematologic Function-Chemotherapy-associated myelosuppression and neutropenic infections increase with age. US and European guidelines endorse prophylactic myeloid growth factors and treatment of anemia in elderly patients receiving moderately myelosuppressive regimens.[63-65]
• Cardiac Toxicity-Age is a known risk factor for anthracycline-associated cardiac toxicity, but several adjuvant trials have shown no increase in age-related risk, possibly due to patient selection.[66-68] Analysis of women over 65 years old in the SEER database, however, shows that women treated with chemotherapy have higher rates of cardiomyopathy (HR = 2.48, CI = 2.10-2.93) and congestive heart failure, despite having a low baseline rate of cardiac disease.[68] Baseline cardiac function should be obtained in all women-especially older women-before using an anthracycline.
• Cognition-In a small study of women aged 65 years and older with early breast cancer, patients had neuro-cognitive testing before and after 6 months of chemotherapy. Cognitive deficits following chemotherapy were seen in 41% of these women. However, 48% of women had unchanged cognitive function, and 11% improved (P = .05). Functional ability, as measured by activities of daily living (ADLs), instrumental activities of daily living (IADLs), and performance status, was unchanged.[69] Further data in this important area are needed.
• Anthracyclines vs CMF vs Taxanes-For older women, the added benefit of anthracycline-containing regimens seen in the overview may be offset by increased toxicity. Compared to CMF, anthracycline-based regimens increase overall and chemotherapy-associated hospital admissions, episodes of febrile neutropenia, and grade 3/4 toxicities.[70,71] In some studies, CMF causes more myelosuppression and associated treatment delays than anthracycline-containing regimens, with a further increase in the incidence of grade 3 mucosal and hematologic toxicity.[72,73]
In a review of CMF-associated toxicity in nine IBCSG studies, an increased mortality rate was seen in older women, primarily associated with sepsis and chemotherapy-associated toxicity.[74] A study comparing CMF to weekly docetaxel (Taxotere) in 153 women who were not eligible for anthracyclines, 137 of whom were over 65, showed higher rates of grade 3/4 neutropenia for CMF but more anemia and dose reductions with docetaxel.[75]
Monoclonal Antibodies
Trastuzumab (Herceptin), an anti-HER2 monoclonal antibody has recently been shown to decrease recurrence risk by about 50% when added to chemotherapy in patients whose tumors are HER2-positive.[76,77] Although HER2 overexpression decreases with age, trastuzumab has few side effects and may be a good option in older patients with good baseline cardiac function and high-risk HER2-positive tumors.[78] Close cardiac monitoring is essential for all patients receiving trastuzumab.
Clinical Trials
Elderly women are underrepresented in clinical trials, despite studies showing that older women are as interested in trial participation as younger women.[79-81] In some cases, underrepresentation is appropriate based on patient comorbidity or unsuitability of available trials. However, several studies suggest that age bias plays a major role in the physician's decision to offer patients trial participation.[82,83]. Enrollment in clinical trials can be complicated and time-consuming but offers older women the best available current treatments and will ultimately improve the evidence base for the treatment of older women with early breast cancer.[84]
In addition to general studies of adjuvant treatment for which many healthy elderly women are eligible, there are currently several cooperative group trials specifically designed for women aged 65 years and older. The Cancer Trials Support Unit/CALGB 49907 trial is comparing the efficacy and toxicity of oral capecitabine (Xeloda) to standard chemotherapy (CMF or AC [doxorubicin (Adriamycin), cyclophosphamide]) in a phase III trial, which will also evaluate quality of life and compliance. Women at least 65 years old with early breast cancer and a primary tumor greater than 1 cm are eligible, regardless of hormone receptor or nodal status. Eligible women who decline this trial can enroll in the companion study, CALGB 369901, which uses a series of questionnaires to evaluate patient preferences regarding chemotherapy, outcome, and quality of life.
In Europe, the Breast International Group (BIG) has two trials of adjuvant chemotherapy in women over 65 years old with early breast cancer. BIG 4-04, or the ICE (Ibandronate with or without Capecitabine in Elderly patients with early breast cancer) study is available for node-positive or high-risk node-negative (tumors greater than 2 cm or hormone receptor-negative) women. Women will receive ibandronate (Boniva), a bisphosphonate that can be given orally or intravenously, and randomized to capecitabine or no further treatment. Outcomes will be correlated with patient preference for oral or intravenous bisphosphonate, creatinine clearance, albumin, hemoglobin, and geriatric assessment scores.
Another option for hormone receptor-negative patients is the BIG 1-05 trial, which will randomize women 65 and older who are not anthracycline candidates to either pegylated liposomal doxorubicin (Doxil) or no treatment, or to pegylated liposomal doxorubicin or low-dose oral cyclophosphamide and methotrexate.
Summary
The treatment of early breast cancer in the elderly is an issue of special concern for the practicing oncologist. The risks and benefits of adjuvant treatment differ in older women, and decision-making can be complicated. Healthy older women should be offered currently available and effective surgical, radiotherapeutic, and systemic treatment options, including clinical trial enrollment. Age alone should not dictate treatment.
Disclosures:
Dr. Muss is a consultant for a Pfizer DSMB, Ortho Biotech, Genentech, and Amgen; has ownership interest in Amgen; has received research grants from AstraZeneca, Aventis, Bristol-Myers Squibb, Merck, GlaxoSmithKline, Ortho Biotech/Tibotech, Aureon, Celgene, Coley, Genentech, Genetics Institute, ImClone, Ligand, Lilly, Novartis, Pfizer, Sandoz, and Schering; fellowship support from Ortho, Amgen, Sanofi-Aventis, and MGI; honoraria from Network Oncology Communication, Neil Love Communications, American Pharmaceutical, and Meditech Ltd; and is on the board of directors and advisory committees of the American Society of Clinical Oncology; and has given expert testimony for RMF/Harvard Medical. Dr. Witherby is a stockholder/has a financial relationship with Johnson & Johnson and Amgen.
References:
1. Jemal A, Murray T, Ward E, et al: Cancer statistics. CA Cancer J Clin 55:10-30, 2005.
2. US Cancer Statistics Working Group. United States Cancer Statistics:1998-2002, Incidence and Mortality, Web-Based Report Version. Atlanta; Department of Health and Human Services, Centers for Disease Control Prevention, and National Cancer Institute; 2005. Available at www.cdc.gov/cancer/npcr/uscs. Accessed July 11, 2006.
3. Public Health and Aging: United States and worldwide. JAMA 289:1371-1373, 2003.
4. Eppenberger-Castori S, Moore DH Jr, Thor AD, et al: Age-associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol 34:1318-1330, 2002.
5. Diab SG, Elledge RM, Clark GM: Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92:550-556, 2000.
6. Basche M, Byers T: Re: Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 93:64-65, 2001.
7. Weiss NS: Re: Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 93:65-66, 2001.
8. Singh R, Hellman S, Heimann R: The natural history of breast carcinoma in the elderly: Implications for screening and treatment. Cancer 100:1807-1813, 2004.
9. Satariano WA, Ragland DR: The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med 120:104-110. 1994.
10. Yancik R, Wesley MN, Ries LA, et al: Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 285:885-892, 2001.
11. Carey EC, Walter LC, Lindquist K, et al: Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med 19:1027-1033, 2004.
12. Extermann M, Overcash J, Lyman GH, et al: Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16:1582-1587, 1998.
13. Extermann M, Aapro M, Bernabei R, et al: Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55:241-252, 2005.
14. Extermann M, Meyer J, McGinnis M, et al: A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol 49:69-75, 2004.
15. Extermann M: Measurement and impact of comorbidity in older cancer patients. Hem Onc Clin North Am 35:181-200, 2000.
16. Wanebo HJ, Cole B, Chung M, et al: Is surgical management compromised in elderly patients with breast cancer? Ann Surg 225:579-586, 1997.
17. Singletary SE, Shallenberger R, Guinee VF: Breast cancer in the elderly. Ann Surg 218:667-671, 1993.
18. Veronesi U, Cascinelli N, Mariani L, et al: Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 349:546-553, 2003.
19. Fisher B, Anderson S, Bryant J, et al: Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233-1241, 2002.
20. Cutuli B, Aristei C, Martin C, et al: Breast conserving therapy for stage I-II breast cancer in elderly women. Int J Radiat Oncol Biol Phys 60:71-76, 2004.
21. Grube BJ, Hansen NM, Wei Y, et al: Surgical management of breast cancer in the elderly patients. Am J Surg 182:359-364, 2001.
22. de Haes JCJM, Curran D, Aaronson NK, et al: Quality of life in breast cancer patients aged over 70 years, participating in the EORTC 10850 randomised clinical trial. Eur J Cancer 39:945-951, 2003.
23. O'Connell JB, Maggard MA, Ko CY: Cancer-directed surgery for localized disease: decreased use in the elderly. Ann Surg Oncol 11:962-969, 2004.
24. Morrow W, White J, Moughan J, et al: Factors predicting the use of breast conserving therapy in stage I and II breast carcinoma. J Clin Oncol 19:2254-2264, 2001.
25. Audisio RA, Bozzetti F, Gennari R, et al: The surgical management of elderly cancer patients: Recommendations of the SIOG task force. Eur J Cancer 40:926-938, 2004.
26. Mandelblatt JS, Kerner JF, Hadley J, et al: Variations in breast carcinoma treatment in older medicare beneficiaries: Is it black or white? Cancer 95:1401-1414, 2002.
27. Martelli G, Miceli R, De Palo G, et al: Is axillary lymph node dissection necessary in elderly patients with breast carcinoma who have a clinically uninvolved axailla. Cancer 97:1156-1163, 2003.
28. Gennari R, Rotmensz N, Perego E, et al: Sentinel node biopsy in elderly breast cancer patients. Surg Oncol 13:193-196, 2004.
29. Bear HD, Anderson S, Brown A, et al: The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: Preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21:4165-4174, 2003.
30. Eiermann W, Paepke S, Appfelstaedt J, et al: Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12:1527-1532, 2001.
31. Deutsch M: Radiotherapy after lumpectomy in very old women. Am J Clin Oncol 25:48, 2002.
32. Vinh-Hung V, Verschaegen C: Breast-conserving surgery with or without radiotherapy: Pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 96:115-121, 2004.
33. Fyles AW, McCready DR, Manchul LA, et al: Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 351:963-970, 2004.
34. Hughes KS, Schnaper LA, Berry D, et al: Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 351:971-977, 2004.
35. Truong PT, Lee J, Kader HA, et al: Locoregional recurrence risks in elderly breast cancer patients treated with mastectomy without adjuvant radiation therapy. Eur J Cancer 41:1267-1277, 2005.
36. Akhtar SS, Allan SG, Rodger A: A 10-year experience of tamoxifen as primary treatment of breast cancer in 100 elderly and frail patients. Eur J Surg Oncol 17:30-35, 1991.
37. Fentiman IS, Christiaens MR, Paridaens R, et al: Treatment of operable breast cancer in the elderly: A randomised clinical trial EORTC 10851 comparing tamoxifen alone with modified radical mastectomy. Eur J Cancer 39:309-316, 2003.
38. Robertson JF, Ellis IO, Elston CW, et al: Mastectomy or tamoxifen as initial therapy for operable breast cancer in elderly patients: 5-year follow-up. Eur J Cancer 28A:908-910, 1992.
39. Gazet JC, Ford HT, Coombes RC, et al: Prospective randomized trial of tamoxifen vs surgery in elderly patients with breast cancer. Eur J Surg Oncol 20:207-214, 1994.
40. Mustacchi G, Ceccherini R, Milani S, et al: Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: Long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol 14:414-420, 2003.
41. Fennessy M, Bates T, MacRae K, et al: Late follow-up of a randomized trial of surgery plus tamoxifen versus tamoxifen alone in women aged over 70 years with operable breast cancer. Br J Surg 91:699-704, 2004.
42. Ellis MJ, Coop A, Singh B, et al: Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol 19:3808-3816, 2001.
43. Ravdin PM, Siminoff LA, Davis GJ, et al: Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 19:980-991, 2001.
44. Extermann M, Balducci L, Lyman GH, et al: What threshold for adjuvant chemotherapy in older breast cancer patients? J Clin Oncol 18:1709-1717, 2000.
45. Early Breast Cancer Trialists' Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005.
46. Polychemotherapy for early breast cancer: An overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 352:930-942, 1998.
47. Goss PE, Ingle JN, Martino S, et al: Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262-1271, 2005.
48. Baum M, Buzdar AU, Cuzick J, et al, for the ATAC Trialists Group: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 359:2131-2139, 2002.
49. Howell A, Cuzick J, Baum M, et al: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365:60-62, 2005.
50. Coombes RC, Hall E, Gibson LJ, et al: A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081-1092, 2004.
51. Jakesz R, Jonat W, Ganat M, et al: Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366:455-462, 2005.
52. Hillner BE, Ingle JN, Chlebowski RT, et al: American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21:4042-4057, 2003.
53. Desch CE, Hillner BE, Smith TJ, et al: Should the elderly receive chemotherapy for node-negative breast cancer? A cost-effectiveness analysis examining total and active life-expectancy outcomes. J Clin Oncol 11:777-782, 1993.
54. Crivellari D, Bonetti M, Castiglione-Gertsch M, et al: Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: The International Breast Cancer Study Group Trial VII. J Clin Oncol 18:1412-1422, 2000.
55. Fisher B, Jeong JH, Anderson S, et al: Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: Updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst 96:1823-1831, 2004.
56. Fisher B, Jeong JH, Bryant J, et al: Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 364:858-868, 2004.
57. Fargeot P, Bonneterre J, Roche H, et al: Disease-free survival advantage of weekly epirubicin plus tamoxifen versus tamoxifen alone as adjuvant treatment of operable, node-positive, elderly breast cancer patients: 6-year follow-up results of the French adjuvant study group 08 trial. J Clin Oncol 22:4622-4630, 2004.
58. Muss HB, Woolf S, Berry D, et al: Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA 293:1073-1081, 2005.
59. Lichtman SM, Skirvin JA: Pharmacology of antineoplastic agents in older cancer patients. Oncology (Williston Park) 14:1743-1755, 2000.
60. Shapiro CL, Recht A: Drug therapy: Side effects of adjuvant treatment of breast cancer. N Engl J Med 344:1997-2008, 2001.
61. Dees EC, O'Reilly S, Goodman SN, et al: A prospective, pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest 18:521-529, 2000.
62. Gelman RS, Taylor SG: Cyclophosphamide, methotrexate and 5-fluorouracil chemotherapy in women more than 65 years old with advanced breast cancer: The elimination of age trends with doses based on creatinine clearance. J Clin Oncol 2:1406-1414, 1984.
63. Balducci L, Lyman GH, Ozer W: Patients aged > or = 70 are at high risk for neutropenic infection and should receive hematopoietic growth factors when treated with moderately toxic chemotherapy. J Clin Oncol 19:1583-1585, 2001.
64. National Comprehensive Cancer Network: Guidelines for supportive care: Myeloid growth factors, version 1.2006. Available at www.nccn.org. Accessed July 12, 2006.
65. Lyman GH, Kuderer N, Agboola O, et al: Evidence-based use of colony-stimulating factors in elderly cancer patients. Cancer Control 10:487-499, 2003.
66. Von Hoff DD, Layard MW, Basa P, et al: Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91:710-717, 1979.
67. Kimmick GG, Shelton BJ, Case LD, et al: Long-term follow up of a phase II trial studying a weekly doxorubicin-based multiple drug adjuvant therapy for stage II node-positive carcinoma of the breast. Breast Cancer Res Treat 72:233-243 2002.
68. Doyle JJ, Neugut AI, Jacobson JJ, et al: Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study (abstract 6085). Breast Cancer Res Treat 94(suppl 1):S278, 2005.
69. Hurria A, Rosen C, Zuckerman E, et al: Effect of adjuvant chemotherapy (CRx) on the cognitive function of older patients (pts) with breast cancer (BC): Results from a prospective study (abstract 8204). Proc Am Soc Clin Oncol 23(16S):779s, 2005.
70. Du XL, Osborne C, Goodwin JS: Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 20:4636-4642, 2002.
71. Hurria A, Brogan K, Panageas KS, et al: Pattern of toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat 92:151-156, 2005.
72. De Maio E, Gravina A, Pacilio C, et al: Compliance and toxicity of adjuvant CMF in elderly breast cancer patients: A single center experience. BMC Cancer 5:30, 2005.
73. Crivellari D, Bonetti M, Castiglione-Gertsch M, et al: Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluoruracil and tamoxifen for elderly patients with breast cancer: The International Breast Cancer Study Group Trial VII. J Clin Oncol 18:1412-1422, 2000.
74. Colleoni M, Price KN, Castiglione-Gertsch M, et al: Mortality during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil. International Breast Cancer Study Group. Lancet 354:130-131, 1999.
75. Yardley DA, Loesch DM, Greco FA, et al: Preliminary toxicity results from a phase III multi-centered trial comparing weekly docetaxel to CMF as adjuvant treatment for high risk breast cancer patients who are not candidates for anthracyclines. (abstract 1061). Breast Cancer Res Treat 88(suppl 1):S62, 2004.
76. Piccart-Gebhart MJ, Proctor M, Leyland-Jones B, et al: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659-1672, 2005.
77. Romond EH, Perez EA, Bryant J, et al: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673-1684, 2005.
78. The HERA study team. Trastuzumab (H: Herceptin) following adjuvant chemotherapy (CT) significantly improves disease-free survival (DFS) in early breast cancer with HER2 overexpression: The HERA trial (abstract 11). Breast Cancer Res Treat 94(suppl 1):S9, 2005.
79. Hutchins LF, Unger JM, Crowley JJ, et al: Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341:2061-2067, 1999.
80. Kemeny MM, Peterson BL, Kornblith AB, et al: Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 21:2268-2275, 2003.
81. Lara PN Jr, Higdon R, Lim N, et al: Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol 19:1728-1733, 2001.
82. Siminoff LA, Zhang A, Saunders Sturm CM, et al: Referral of breast cancer patients to medical oncologists after initial surgical management [see comments]. Med Care 38:696-704, 2000.
83. Kemeny M, Muss HB, Kornblith AB, et al: Barriers to participation of older women with breast cancer in clinical trials (abstract 2371). Proc Am Soc Clin Oncol 19:602a, 2000.
84. Kornblith AB, Kemeny M, Peterson BL, et al: Survey of oncologists' perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer 95:989-996, 2002.
85. Schairer C, Mink PJ, Carroll L, et al: Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst 96:1311-1321, 2004.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.