MBCC: Treatment Targets for Triple-Negative Breast Cancer-Three Pathways to Test in the Clinic
What are the latest advances in the treatment of triple-negative breast cancer? Are there new ways to molecularly characterize breast cancer tumors to identify specific mutation targets and increase the chance of response in this disease?
What are the latest advances in the treatment of triple-negative breast cancer? Are there new ways to molecularly characterize breast cancer tumors to identify specific mutation targets and increase the chance of response in this disease?
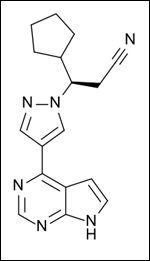
Chemical Structure of ruxolitinib, a JAK2 inhibitor that is currently part of an ongoing trial at the Dana-Farber Cancer Institute
These are the topics discussed on the second day of the 29th Annual Miami Breast Cancer Conference.
"We need to formulate hypotheses, and then test these in the clinic because triple-negative disease is still a highly unmet medical need," said Dr. Joyce O'Shaughnessy, energetically, during the introduction to her overview on the current emerging therapies for triple-negative breast cancer.
Breast cancer is already treated based on molecular subtypes-HER2-positive, estrogen-receptor positive, and progesterone-receptor positive. But triple-negative breast cancer is only defined by what mutations it lacks. Triple-negative breast cancer accounts for 15% to 20% of all breast cancers. One-third of these patients develop advanced, metastatic disease despite advances in adjuvant chemotherapies.
While three chemotherapy "doublets" have been validated in phase III clinical trials (see CancerNetwork's podcast with Dr. O'Shaughnessy), many patients relapse, and identifying new targetable mutations for this disease is one of the keys for future therapies.
Crippling the Cancer With More DNA Damage
Basal triple-negative breast cancer cells, according to O'Shaughnessy, have defects in homologous recombination, including mutations in the genes ATM and RAD51. The current hypothesis is that overwhelming the DNA of cancer cells with DNA damaging agents or inhibiting pathways that can compensate for a lack of repair will result in better tumor responses.
The problem, said O'Shaughnessy, is identifying the triple-negative breast cancer patients that have these repair defects. One potential way to identify patients with the repair defects, as suggested by the speaker, is to make more use of an array-based comparative genomic hybridization, which can identify genomic instability-copy number fluctuations, deletions, and rearrangements. "It is an interesting hypothesis, to exploit the cancer's problem with homologous recombination," she said.
Three Pathways at Play in Triple-Negative Breast Cancer
Besides homologous recombination, triple-negative breast cancers also harbor mutations in well-defined pathways with available agents that target these mutations.
O'Shaughnessy highlighted that the MAPK and AKT/PI3K pathways are coactivated in at least some metastatic triple-negative breast cancers, and at least ten phase I clinical trials are ongoing, testing an MEK inhibitor plus an AKT inhibitor, both involved in these pathways, to address whether targeting these pathways is a useful approach in this indication. However, more analyses of the effect of these mutations on cancer growth and the interaction of the mutations with other pathways are necessary to identify potential combination therapies.
Another "intriguing" target that O'Shaughnessy described is the epidermal growth factor receptor (EGFR), which is "one of the defining proteins that characterizes the basal-like subset of triple-negative breast cancer."
O'Shaughnessy would like to see a clinical trial that specifically targets the patients with an EGFR mutation, which has not yet been done. She pointed out that the BALI-1 trial from 2010 testing the EGFR antibody, cetuximab, in combination with cisplatin in metastatic triple-negative breast cancer patients. The trial showed a modest improvement in progression-free survival, but did not specifically target patients with an EGFR genetic aberration, which could be why the primary end point of the trial was not reached.
A third pathway highlighted during the presentation was the JAK2/STAT3 pathway. O'Shaughnessy cited a recent observation that when tumor samples from patients were assayed for a STAT3, a transcription factor, localization of STAT3 to the nucleus correlated with a worse outcome in patients. There is currently an ongoing trial of a JAK2 inhibitor, ruxolitinib, at the Dana-Farber Cancer Institute in Boston, Mass.
In conclusion, Dr. O'Shaughnessy emphasized that there are promising targets for basal breast cancers-homologous recombination and EGFR, as well as data that validates targeting the STAT3/JAK2 pathway, a "key signaling pathway," and that the way forward is with clear hypotheses that can be tested in the clinic.
A major benefit of testing these new drugs in clinical trials is to understand the mechanisms of resistance. In a session on PARP inhibitors, Dr. Jorge S. Reis-Filho outlined three ways that resistance can occur in patients who are treated with PARP inhibitors, including a functional restoration of BRCA1 or BRCA2 mutations, stressing that the process is multifactorial.
Understanding these processes by honing in on specific patients with specific genetic aberrations is likely to be beneficial for developing better treatments and combinations of treatments.