Molecular Targets on Blood Vessels for Cancer Therapies in Clinical Trials
This review covers progress to date in the identification of molecular targets on blood vessels in cancers, as well as agents that act on those targets, with emphasis on those currently in clinical trials. Current vascular-targeting therapies comprise two general types—antiangiogenic therapy and antivascular therapy. Advances in antiangiogenic therapies, particularly inhibitors of vascular endothelial growth factors and their receptors, have clarified the capacity of these inhibitors to change tumor-associated vessel structure to a more normal state, thereby improving the ability of chemotherapeutics to access the tumors. The responses of other antiangiogenesis target molecules in humans are more complicated; for example, αvβ3 integrins are known to stimulate as well as inhibit angiogenesis, and cleavage of various extracellular proteins/proteoglycans by matrix metalloproteinases produces potent regulators of the angiogenic process. Antivascular therapies disrupt established blood vessels in solid tumors and often involve the use of ligand-based or small-molecule agents. Ligand-based agents, irrespective of the antiangiogenic capacity of the ligand, target antivascular effectors to molecules expressed specifically on blood vessels, such as aminopeptidase N, fibronectin extra-domain B, and prostate-specific membrane antigen. Small-molecule antivascular agents, which are not targeted to molecules on blood vessels, rely on physical differences between the vasculatures in tumors and those in normal tissues.
This review covers progress to date in the identification of molecular targets on blood vessels in cancers, as well as agents that act on those targets, with emphasis on those currently in clinical trials. Current vascular-targeting therapies comprise two general types-antiangiogenic therapy and antivascular therapy. Advances in antiangiogenic therapies, particularly inhibitors of vascular endothelial growth factors and their receptors, have clarified the capacity of these inhibitors to change tumor-associated vessel structure to a more normal state, thereby improving the ability of chemotherapeutics to access the tumors. The responses of other antiangiogenesis target molecules in humans are more complicated; for example, αvβ3 integrins are known to stimulate as well as inhibit angiogenesis, and cleavage of various extracellular proteins/proteoglycans by matrix metalloproteinases produces potent regulators of the angiogenic process. Antivascular therapies disrupt established blood vessels in solid tumors and often involve the use of ligand-based or small-molecule agents. Ligand-based agents, irrespective of the antiangiogenic capacity of the ligand, target antivascular effectors to molecules expressed specifically on blood vessels, such as aminopeptidase N, fibronectin extra-domain B, and prostate-specific membrane antigen. Small-molecule antivascular agents, which are not targeted to molecules on blood vessels, rely on physical differences between the vasculatures in tumors and those in normal tissues.
Targeting therapeutic agents specifically to disease lesions, as opposed to systemic delivery, can reduce toxicity and enhance therapeutic efficacy.[1] Despite these advantages, such specificity remains an elusive goal in molecular medicine. The tumor vasculature is an attractive target in anticancer therapy for several reasons: (1) it is easily accessed by a variety of therapeutic molecules, (2) it represents a vital route for delivery of oxygen and other nutrients essential for tumor growth, (3) it is one of the main routes of metastatic spread, (4) the genetic stability of vascular endothelial cells (in comparison with tumor cells) makes them attractive targets for long-term therapy, and (5) tumor vasculature–specific selection markers should make this form of treatment applicable to a range of solid tumors. Thus, extensive effort has been devoted to the discovery of novel therapeutic agents that can target the tumor vasculature.
Current vascular-targeting therapies are of two general types: antiangiogenic agents, which block endothelial cell survival and prevent the supply of blood to tumors, and antivascular agents, which disrupt established blood vessels in solid tumors. Two types of antivascular agents are currently being studied: ligand-directed agents and other small molecules. Success in animal models targeting a variety of molecules expressed on blood vessels within tumors has led to testing of several vascular-targeting agents in clinical trials. Here we review progress in the identification of molecular targets on vasculature in cancers, as well as agents that act on those targets, with particular emphasis on those currently in clinical trials.
Target Molecules for Antiangiogenesis Therapy
Most of the molecules identified as targets on tumor blood vessels are associated with angiogenesis and stromal reorganization. Moreover, many are expressed by endothelial cells, and some are produced by the tumor cells themselves, by pericytes, or by components of the extracellular matrix. A variety of vascular-targeting agents that affect cancer through the inhibition of angiogenesis are currently in clinical trials (Table 1) and are reviewed in the following paragraphs.
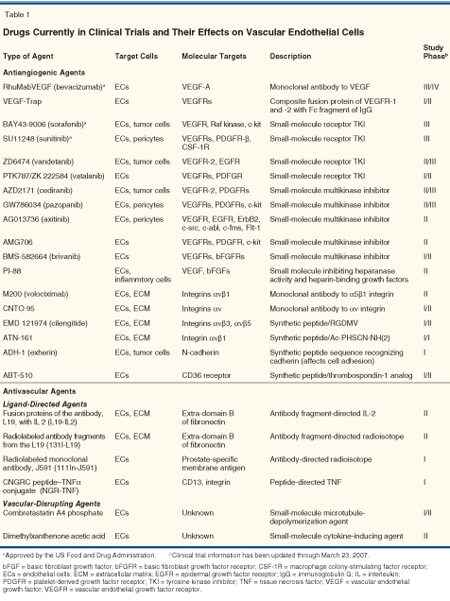
Vascular Endothelial Growth Factors and Their Receptors
The main targets of many antiangiogenic drugs are the vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs). A single administration of the humanized monoclonal antibody against VEGF, bevacizumab (Avastin), decreased tumor perfusion, vascular volume, microvascular density, and interstitial pressure within tumors of rectal carcinoma patients.[2] Batchelor et al recently reported that daily doses of cediranib (AZD2171), a tyrosine kinase inhibitor of VEGFRs, led to decreases of vessel size and blood flow in tumors, and alleviation of edema in patients with recurrent glioblastoma.[3] Given in combination with standard chemotherapy, bevacizumab has been shown to prolong survival in patients with metastatic colon, breast, and lung cancer.[4,5]
These findings support the concept that inhibition of VEGF transiently changes atypical vascular structure and permeability to a more normal state, effects that reduce interstitial pressure within tumors and thereby improve the access of oxygen or chemotherapeutics to the tumors.[6] Therefore, it is indicated that anti–VEGF-signaling agents should be combined with cytotoxic chemotherapy to induce a tumor response sufficient for improved survival of patients. Alternatively, in addition to the blocking of VEGF signaling, inhibition of other growth factor signaling pathways by small-molecule multikinase inhibitors (Table 1) would be required. Indeed, treatment with sorafenib (BAY 43-9006, Nexavar), prolongs progression-free survival in patients with advanced renal cell carcinoma.[7,8]
Integrins
Integrins mediate cellular attach ment to the extracellular matrix and subsequent signal transduction. Many types of integrins-eg, αvβ3, αvβ5, α5β1, and α6β4-are enhanced on growing endothelial cells and/or tumor cells, and on cancer-associated but not mature endothelia. Such observations have indicated their potential in the targeting of both endothelial and tumor cells.[9] Systemic administration of anti-αvβ3 antibody or peptide inhibitors of αvβ3 integrin has been shown to block tumor angiogenesis and to reduce the growth and invasiveness of human tumors in animals.[10,11] However, findings from mouse models of genetic ablation were less clear-eg, angiogenesis was not blocked in mice lacking β3, β5, or β6 integrin. Moreover, angiogenesis was enhanced in mice lacking αv integrin, results indicating that αvβ3 integrin could have both positive and negative regulatory roles in various phases of angiogenesis.[12] Phase II trials are currently underway to test a humanized anti-αvβ3 antibody (Abegrin, formerly known as Vitaxin) for stage IV metastatic melanoma and androgen-independent prostate cancer that metastasizes to bone.[11]
The αvβ3 integrin serves as a receptor for a variety of extracellular matrix proteins, including vitronectin, fibronectin, fibrinogen, thrombospondin 1, laminin, and several collagens, via their exposed arginine-glycine-aspartic acid (RGD) tripeptide sequence.[13,14] Cyclic RGD-containing peptides are the most commonly studied integrin inhibitors. One such peptide, cilengitide (EMD 121974, an N-methylated form of cRGDfV), selectively inhibits the binding of vitronectin to the αvβ3 receptor. In preclinical mouse models, daily administration of cilengitide led to shrinkage of orthotopically-implanted human brain tumor cells in mice and prolonged the ir survival.[15] Phase II studies of cilengitide for patients with glioblastoma or prostate cancer are ongoing.
Our group has developed a novel vehicle for gene therapy that consists of a chimerized adeno-associated virus and single-stranded bacteriophage (AAVP). The prototype was confirmed to target αv integrin exposed on the epithelial cell surface and to inhibit tumor growth in mice.[16]
Matrix Metalloproteinases
Previously, matrix metalloproteinases (MMPs) were thought to act primarily by their promotion of the migration or invasion of tumor cells, stromal cells, or epithelial cells via degradation or reorganization of tumor-associated stroma and basement membrane. These characteristics make MMPs attractive targets for treatment of late-stage (metastatic) disease. However, phase III trials of broad-spectrum MMP inhibitors for the most part have not resulted in extended survival, because the effects of MMPs are more complex than were anticipated.
Proteolysis by MMPs regulates aspects of cell signaling that control the homeostasis of the extracellular environment.[17] For example, cleavage of various substrates by MMPs can give rise to angiostatin and endostatin, both potent inhibitors of angiogenesis.[17] Therefore, selecting particular MMPs as targets in the treatment of cancer will require the knowledge that a particular MMP acts specifically on the target (and not on related molecules that could be considered "antitargets," ie, molecules that could have deleterious effects if blocked or disrupted).
Our work in this regard focuses on MMP-2 and MMP-9, which are present on the tumor epithelium and in the stroma of several types of cancer, including that of breast, colon, skin, and lung.[18] We showed that a cyclic peptide containing the sequence HWGF, isolated from in vivo phage display screening, selectively inhibits MMP-2 and MMP-9 but not several other MMPs.[19] Moreover, our prototype synthetic peptide CTTHWGFTLC has been shown to prevent tumor growth and invasion in animal models, to improve survival of mice bearing human tumors, and to block migration of endothelial cells.
Aminopeptidase N
Aminopeptidase N (APN, also known as CD13) is distributed widely, yet it plays tissue-specific roles that depend on differential cleavage of various biologically active peptides such as enkephalins, endorphins, and angiotensins.[20] We found APN to be expressed exclusively on endothelial cells in mouse and human tissues undergoing angiogenesis, but not on those in the absence of angiogenesis.[21,22] Others have shown that 61% of 62 clinical samples of squamous cell carcinomas of the lung showed expression of APN in interstitial cells, including fibroblasts and endothelial cells, but not in the tumor cells themselves.[23] Mice deficient in the APN gene grow normally but exhibit an impaired angiogenic response under pathologic conditions.[24]
APN antagonists such as anti-APN antibodies and bestatin have shown antiangiogenic effects and have led to reduced tumor volume in breast cancer models.[22] APN is therefore an attractive target, not only for antiangiogenic therapy but also attractive for ligand-based therapy. In comparison to αvβ3 integrin, we have shown that APN is an alternative and more specific binding target than for peptides containing the NGR motif.[22] Fusing the anticancer agent tumor necrosis factor (TNF) with the peptide CNGRC, an APN ligand efficiently targets activated blood vessels and shows antitumor effects in murine models.[25] This ligand-directed drug is currently in phase I trials (Table 1).
Vascular Target Molecules for Ligand-Based Antivascular Therapy
In addition to VEGFs, VEGFRs, integrins, MMPs, and APN, other molecules on epithelial cells have also been tested for their capacity to block angiogenesis in tumors. Such molecules can be used not only as antiangiogenic effectors, but also as targets for other types of effectors.[26] Molecules expressed specifically on endothelial cells that have been used for research on ligand-directed vascular-targeted therapy are shown in Table 2. Several ligand-directed agents targeting molecules such as phosphatidylserine, a CD44 isoform (CD44H), and annexin I have shown promising results in animal models but have yet to reach clinical trials.[27] Targeting molecules for ligand-directed agents in clinical trials are described below.
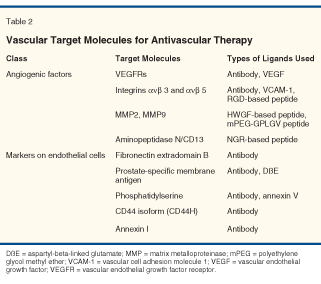
Fibronectin Extra-Domain B
Fibronectin is a large glycoprotein abundant in tissues and plasma. Tissues undergoing growth or remodeling express the extra-domain B of fibronectin, an isoform created by alternative splicing. Fibronectin containing extra-domain B accumulates around blood vessels in aggressive solid human tumors and in other tissues undergoing angiogenesis, but this isoform has not been detected in vessels and tissues lacking angiogenesis.[27] A dimeric form of the scFv fragments in L19, a high-affinity human recombinant antibody specific for extra-domain B, conjugated to the 123I has been shown to target lung, colorectal, or brain cancer selectively in human subjects.[27]
Many effector derivatives of the L19 antibody and the scFv fragments have been examined in animal models. For example, fusion proteins of L19 with interleukin 2 (L19-IL2), interleukin 12, TNF, or interferon-? have exhibited antitumor activity in mice.[28] L19-IL2 and an 131I-radioconjugated derivative of scFv fragments are currently in clinical trials for cancer therapy.[29]
Prostate-Specific Membrane Antigen
Prostate-specific membrane antigen (PSMA), a transmembrane carboxypeptidase, is restricted largely to prostatic epithelial cells in humans and is strongly enhanced on prostate carcinoma cells. Radioimmunotherapy with the monoclonal anti-PSMA antibody J591 has progressed to phase I trials for the treatment of prostate cancer.[30,31] PSMA is also expressed on the neovascular endothelium of various solid tumors but not on tumor cells themselves or vascular endothelium in nonmalignant tissues.[32] In one preclinical study, functional targeting of coagulation-inducing tissue factor to PSMA on microvascular lining cells resulted in vascular thrombosis and tumor necrosis in tumor-bearing mice.[33] PSMA is thus considered an encouraging target for ligand-directed vascular therapy as well. In a phase I study of patients with advanced solid tumors, at least one region of known PSMA-expressing metastasis was successfully targeted by 111In J591 in 20 of 27 patients.[34]
Vascular Target Effectors for Antivascular Therapy
A range of molecules has been used as vascular target effectors, such as radioisotopes,[34] cytokines,[28] cytotoxic agents,[1] bacterial toxins,[35] a coagulation-inducing tissue factor,[33] apoptosis-inducing agents,[36,37] and genes[16] (reviewed in reference [26]). Some of the promising target effectors are already in the clinic for cancer cell–targeting therapy. For example, ibritumomab tiuxetan (Zevalin) and tositumomab/131I-tositumomab (Bexxar) are both anti-CD20 monoclonal antibodies linked to radioisotopes, gemtuzumab (Mylotarg) is an anti-CD33 antibody linked to a cytotoxic agent, and denileukin diftitox (Ontak) is an anti-CD25 agent linked to a bacterial toxin. Our work with an apoptosis-inducing peptide is described below.
Apoptosis-Inducing Peptide
The apoptosis-inducing peptide D(KLAKLAK)2 disrupts eukaryotic mitochondrial membranes, as opposed to plasma membranes, and results in apoptosis after internalization under the guidance of a homing peptide.[38] We are currently developing D(KLAKLAK)2 as part of a vascular-disrupting strategy in prostate and adipose tissue. Briefly, we found the in vivo phage display-derived peptide SMSIARL homes to vascular epithelial cells in prostate tissue. Furthermore, the chimeric peptide SMSIARL-GG-D(KLAKLAK)2 caused prostate-specific tissue destruction and delayed the development of prostate cancer in prostate cancer–prone transgenic mice.[36] In another application, we conjugated D(KLAKLAK)2 with CKGGRAKDC, a peptide that homes to vascular endothelial cells of white adipose tissue.[37] Targeting of these vessels with the conjugated peptide disrupted their structure and reversed obesity in a mouse model. These two examples show that combining peptides that home to a specific type of vascular endothelial cell with proapoptotic peptides is a promising approach for disruption of the vasculature in cancers and other tissues.
Small-Molecule Vascular-Disrupting Agents
Two types of small-molecule vascular-disrupting agents, one of which acts by depolymerization of microtubules and the other, by induction of cytokines, disrupt established tumor vasculature. This abrogation blocks the flow of oxygen and other nutrients to the tumor and leads to secondary hemorrhagic necrosis. With these agents, tumor selectivity is conferred, not by specific targeting, but by differences in the pathophysiology of tumor vs normal tissue vasculature (eg, the forms are characterized by greater proliferation and fragility, irregularities in intercellular openings and overlap, and high vascular permeability and internal fluid pressure).[26,39] Two vascular-disrupting agents currently in clinical trials (Table 1) are reviewed in the following paragraphs.
CA-4-P
Combretastatin A-4 disodium phosphate (CA-4-P) is a prodrug of combretastatin A-4, which binds to tubulin and inhibits tubulin polymerization. In one study, administration of CA-4-P almost completely abrogated tumor blood flow within 1 hour and resulted in tumor necrosis.[40] Despite its selective and strong antitumor effects, CA-4-P has not exhibited positive effects on survival to date, perhaps because viable cells that persist in the peripheral rims of the tumors contribute to tumor regrowth.[39] However, given in combination with radiation and chemotherapy, CA-4-P has shown enhanced antitumor effects in experimental models. Moreover, in phase I clinical studies, 3 of 19 patients showed some evidence of an antitumor effect from CA-4-P, including one complete response of anaplastic thyroid cancer[41] and a partial response of metastatic soft-tissue sarcoma.[42] In another phase I study, CA-4-P given in combination with carboplatin led to stabilization of disease for 12 weeks or more in six cases.[43]
DMXAA
5,6-Dimethyxanthenone-4-acetic acid (DMXAA) is 12 times more potent than flavone-8-acetic acid, a compound with exceptional activity against a wide range of solid tumors with established vasculature.[39] Treatment of tumor-bearing mice with DMXAA led to rapid decreases in blood flow within tumors that was followed by tumor necrosis-without thin peripheral rims-within 24 hours. DMXAA activates tumor-associated macrophages to release chemokines and inflammatory cytokines, mainly TNFα, that lead to tumor necrosis and the generation of CD8+ T cells, which are required for the antitumor effects of this compound.[44] Phase I trials conducted to date have reported two unconfirmed partial responses and 28 cases of stable disease (among 109 cases).[45,46] Phase II trials are underway to test the effects of DMXAA in combination with taxanes for the treatment of metastatic prostate cancer.
Summary and Future Directions
Advances in therapy with inhibitors of VEGFs and their receptors have shown that these inhibitors can improve the access of chemotherapeutic agents to tumors. The action of other agents that target molecules involved in angiogenesis in humans (eg, integrins and MMPs) is more complex than was anticipated. Further elucidation of the distinct steps involved in angiogenic signaling will facilitate development of antiangiogenic therapy. Alternatively, small-molecule antivascular agents do not target specific molecules on blood vessels, but rely on physical differences between the vasculatures in tumors and those in normal tissues. Ligand-based agents carry antivascular effectors toward molecules expressed specifically on tumor blood vessels, irrespective of the antiangiogenic capacities of the ligands. It will therefore be important to search for new vascular markers that discriminate the vessels in tumors from those in normal tissues, or those of a particular organ vs another.
Structural heterogeneity and functional differences in the vasculatures of normal organs or tissues, as well as differences between quiescent and activated endothelial cells in blood vessels, are reflected in the molecular diversity of their surface receptors.[47] These molecular differences can also be exploited to target therapeutic agents to a selected organ as well as a tumor. To this end, we have used in vivo phage display to identify peptides or peptide motifs that target the vasculature of normal organs in mice and humans, including brain, kidney, prostate, skin, pancreas, retina, intestine, uterus, adrenal gland, muscle, and pancreatic islets.[48-51] For instance, the phage mimicking IL-11 was found to bind to blood vessels and to the endothelium in tissue sections of human prostate cancers, as well as epithelial glands in hyperplastic and normal prostate, but not to vasculature in other tissues.[49,52] A prolactin-mimicking peptide isolated from a mouse pancreas was shown by immunohistochemical analysis to localize to pancreatic blood vessels and islet cells.[50]
Creation of a human vascular map that includes "addresses" and "ZIP codes" for specific organs would be tremendously useful as the basis for the development and application of targeted therapies, not only for cancers but for other diseases as well.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Arap W, Pasqualini R, Ruoslahti E: Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377-380, 1998.
2. Willett CG, Boucher Y, di Tomaso E, et al: Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145-147, 2004.
3. Batchelor TT, Sorensen AG, di Tomaso E, et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83-95, 2007.
4. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
5. Marx J: Cancer. Encouraging results for second-generation antiangiogenesis drugs. Science 308:1248-1249, 2005.
6. Jain RK: Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58-62, 2005.
7. Motzer RJ, Hutson TE, Tomczak P, et al: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115-124, 2007.
8. Escudier B, Eisen T, Stadler WM, et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125-134, 2007.
9. Brooks PC, Clark RA, Cheresh DA: Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264:569-571, 1994.
10. Hammes HP, Brownlee M, Jonczyk A, et al: Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med 2:529-533, 1996.
11. Mulgrew K, Kinneer K, Yao XT, et al: Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther 5:3122-3129, 2006.
12. Hynes RO: A reevaluation of integrins as regulators of angiogenesis. Nat Med 8:918-921, 2002.
13. Pierschbacher MD, Ruoslahti E: Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309:30-33, 1984.
14. Gardner JM, Hynes RO: Interaction of fibronectin with its receptor on platelets. Cell 42:439-448, 1985.
15. MacDonald TJ, Taga T, Shimada H, et al: Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery 48:151-157, 2001.
16. Hajitou A, Trepel M, Lilley CE, et al: A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 125:385-398, 2006.
17. Overall CM, Kleifeld O: Tumour microenvironment-opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6:227-239, 2006.
18. Nelson AR, Fingleton B, Rothenberg ML, et al: Matrix metalloproteinases: Biologic activity and clinical implications. J Clin Oncol 18:1135-1149, 2000.
19. Koivunen E, Arap W, Valtanen H, et al: Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol 17:768-774, 1999.
20. Luan Y, Xu W: The structure and main functions of aminopeptidase N. Curr Med Chem 14:639-647, 2007.
21. Bhagwat SV, Lahdenranta J, Giordano R, et al: CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 97:652-659, 2001.
22. Pasqualini R, Koivunen E, Kain R, et al: Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res 60:722-727, 2000.
23. Ichimura E, Yamada M, Nishikawa K, et al: Immunohistochemical expression of aminopeptidase N (CD13) in human lung squamous cell carcinomas, with special reference to Bestatin adjuvant therapy. Pathol Int 56:296-300, 2006.
24. Rangel R, Sun Y, Guzman-Rojas L, et al: Impaired angiogenesis in aminopeptidase N-null mice. Proc Natl Acad Sci U S A 104:4588-4593, 2007.
25. Curnis F, Sacchi A, Borgna L, et al: Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat Biotechnol 18:1185-1190, 2000.
26. Thorpe PE: Vascular targeting agents as cancer therapeutics. Clin Cancer Res 10:415-427, 2004.
27. Neri D, Bicknell R: Tumour vascular targeting. Nat Rev Cancer 5:436-446, 2005.
28. Ahlskog J, Paganelli G, Neri D: Vascular tumor targeting. Q J Nucl Med Mol Imaging 50:296-309, 2006.
29. Trachsel E, Kaspar M, Bootz F, et al: A human mAb specific to oncofetal fibronectin selectively targets chronic skin inflammation in vivo. J Invest Dermatol 127:881-886, 2007.
30. Bander NH, Milowsky MI, Nanus DM, et al: Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol 23:4591-4601, 2005.
31. Milowsky MI, Nanus DM, Kostakoglu L, et al: Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol 22:2522-2531, 2004.
32. Liu H, Moy P, Kim S, et al: Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 57:3629-3634, 1997.
33. Liu C, Huang H, Donate F, et al: Prostate-specific membrane antigen directed selective thrombotic infarction of tumors. Cancer Res 62:5470-5475, 2002.
34. Milowsky MI, Nanus DM, Kostakoglu L, et al: Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J Clin Oncol 25:540-547, 2007.
35. Wild R, Yokoyama Y, Dings RP, et al: VEGF-DT385 toxin conjugate inhibits mammary adenocarcinoma development in a transgenic mouse model of spontaneous tumorigenesis. Breast Cancer Res Treat 85:161-171, 2004.
36. Arap W, Haedicke W, Bernasconi M, et al: Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci U S A 99:1527-1531, 2002.
37. Kolonin MG, Saha PK, Chan L, et al: Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10:625-632, 2004.
38. Ellerby HM, Arap W, Ellerby LM, et al: Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med 5:1032-1038, 1999.
39. Tozer GM, Kanthou C, Baguley BC: Disrupting tumour blood vessels. Nat Rev Cancer 5:423-435, 2005.
40. Tozer GM, Prise VE, Wilson J, et al: Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: Intravital microscopy and measurement of vascular permeability. Cancer Res 61:6413-6422, 2001.
41. Dowlati A, Robertson K, Cooney M, et al: A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 62:3408-3416, 2002.
42. Stevenson JP, Rosen M, Sun W, et al: Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 21:4428-4438, 2003.
43. Bilenker JH, Flaherty KT, Rosen M, et al: Phase I trial of combretastatin a-4 phosphate with carboplatin. Clin Cancer Res 11:1527-1533, 2005.
44. Jassar AS, Suzuki E, Kapoor V, et al: Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res 65:11752-11761, 2005.
45. Jameson MB, Thompson PI, Baguley BC, et al: Clinical aspects of a phase I trial of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent. Br J Cancer 88:1844-1850, 2003.
46. Rustin GJ, Galbraith SM, Anderson H, et al: Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol 21:2815-2822. 2003.
47. Pasqualini R, Arap W, McDonald DM: Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med 8:563-571, 2002.
48. Kolonin M, Pasqualini R, Arap W: Molecular addresses in blood vessels as targets for therapy. Curr Opin Chem Biol 5:308-313, 2001.
49. Arap W, Kolonin MG, Trepel M, et al: Steps toward mapping the human vasculature by phage display. Nat Med 8:121-127, 2002.
50. Kolonin MG, Sun J, Do KA, Vidal CI, et al: Synchronous selection of homing peptides for multiple tissues by in vivo phage display. Faseb J 20:979-981, 2006.
51. Yao VJ, Ozawa MG, Trepel M, et al: Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am J Pathol 166:625-636, 2005.
52. Zurita AJ, Troncoso P, Cardo-Vila M, et al: Combinatorial screenings in patients: The interleukin-11 receptor alpha as a candidate target in the progression of human prostate cancer. Cancer Res 64:435-439, 2004.