Recent improvements in our understanding of the biology of colorectal cancer have led to the identification of several important prognostic and predictive markers of disease-associated risk and treatment response for the individual patient. Proper utilization of these biomarkers can enable physicians to tailor therapeutic strategies to maximize the likelihood of response and minimize treatment toxicity. In the management of colorectal cancer, tremendous progress has been made in the development of strategies for immune checkpoint inhibition; in refinement of agents and approaches used in targeted therapy; and in techniques for molecular subtyping of tumor samples that have identified patient subgroups with clinically relevant cellular differences potentially affecting clinical management and treatment outcome. In this article, we discuss several of the commonly tested markers in colorectal cancer-including microsatellite instability, RAS/RAF, DPD, HER2, UTG1A1, TS, and Immunoscore-and highlight their prevalence, prognostic and predictive value, and current role in the overall treatment paradigm.
Introduction
Colorectal cancer is the fourth most common cancer diagnosed in the United States and the second leading cause of cancer-related death. Approximately 140,000 cases are diagnosed annually in the United States, and 1 in 5 patients have metastatic disease at the time of diagnosis.[1] Advances in management of colorectal cancer, especially of metastatic disease, have increased the median overall survival (OS) time from 12 months to more than 30 months,[2] due in large part to gains over the last few years in the development of targeted therapy and molecular testing. Historically, prognostic and predictive markers in colorectal cancer have relied on clinical and pathologic features only. For example, over the last few decades, lymph node positivity and the presence of metastasis were the only prognostic/predictive markers in colorectal cancer.
In contrast, the postgenomic era has been marked by the evolution of tumor molecular profiling, with the discovery of multiple new oncologic markers for use not only with targeted therapy but also with standard cytotoxic therapy. The information gained from tumor testing allows us to begin to move away from the currently employed trial-and-error methods and towards selecting the right patient for the right treatment. In this review, we discuss several of the most commonly tested markers in colorectal cancer and explore the rapidly changing landscape into which oncologists must integrate an ever-increasing array of molecular information. We highlight the new treatment opportunities that molecular testing is beginning to provide, and describe the ways in which clinicians can optimize the use of newly available molecular-based tools in the face of the rising cost of cancer care.
We also discuss the current standard of care for colorectal cancer, which includes testing for the RAS gene and microsatellite instability (MSI)/microsatellite stability (MSS) in tumor cells. We describe other recommended genetic evaluations, including testing for mutations of BRAF, UGT1A1, and DPD, and for amplification of HER2. Recent studies of testing for expression of the TS gene and TS protein, often considered to be confined to the research setting, are also evaluated. Finally, we highlight biomarkers for which there is emerging evidence of clinical relevance in colorectal cancer but currently no widespread adoption into clinical practice, including Immunoscore and consensus molecular subtypes.
MSI/Mismatch Repair (MMR) Status
MMR status should be tested in all patients with colorectal cancer, independent of the initial stage of disease. Microsatellites are short, repetitive nucleotide sequences of variable length that are distributed throughout genomic DNA, and MMR proteins are DNA repair enzymes that correct insertion/deletion loops, as well as base pair mismatches that occur during DNA replication.[3] Approximately 20% of colorectal cancer tumors have MSI, a condition of genetic hypermutability characterized by truncation or expansion of microsatellites caused directly by impaired MMR enzyme activity.[4] Dysfunctional MMR activity may result from a germline mutation in one of the four major MMR genes: MLH1, MSH2, MSH6, and PMS2. Germline MMR gene mutation is observed in 15% to 20% of patients with colorectal cancer; a mutation in any of the four MMR genes represents a defining clinical feature of Lynch syndrome, which occurs in 3% to 5% of cases. Disruption of MMR activity also may occur sporadically (as seen in 80% of colorectal cancer patients) as a consequence of methylation of CpG islands (regions with at least 200 base pairs in which the frequency of the cytosine-guanine sequence is higher than in other regions, with “p” simply indicating that “C” and “G” are connected by a phosphodiester bond) in MMR promoter sequences (most commonly in MLH1).[5,6] A subset of MMR-deficient (MMRD) tumors appears to be sporadically associated with the CpG island methylator phenotype (CIMP). The presence of CIMP-mediated MSI tumors is inversely correlated to that of tumors with the chromosomal instability pathway (CIN) phenotype, suggesting little overlap between the molecular mechanisms underlying CIMP vs CIN; also, CIMP-positive cases tend to have activating mutations in BRAF.[3,4,7]
MMR status can be evaluated by MSI testing, which is typically performed using polymerase chain reaction (PCR) to amplify a standard panel of microsatellite sequences in both tumor and normal tissue from the same patient. A positive result, MSI-high (MSI-H), is defined as expansion or contraction of at least 40% of microsatellite sequences in the tumor compared with normal DNA; a status of MSI-low (MSI-L), or MSS, is considered negative. Alternatively, tumors can be evaluated for expression of four major MMR proteins (MLH1, MSH2, MSH6, and PMS2) using immunohistochemistry (IHC) testing, in which the loss of nuclear expression, or MMRD status, is closely correlated with having an MSI-H status.[6] IHC results of MMRD and/or MSI-H typically warrant PCR-based testing for germline mutations, to determine whether the patient has Lynch syndrome.[4] The sensitivity of IHC and PCR testing methods is similar; however, MMR testing using IHC is more practical and less expensive.[6] Algorithms are evolving to include testing for somatic BRAF mutations and MLH1 promoter hypermethylation, the presence of which effectively precludes the need for testing tumor samples for germline mutations associated with Lynch syndrome.[8]
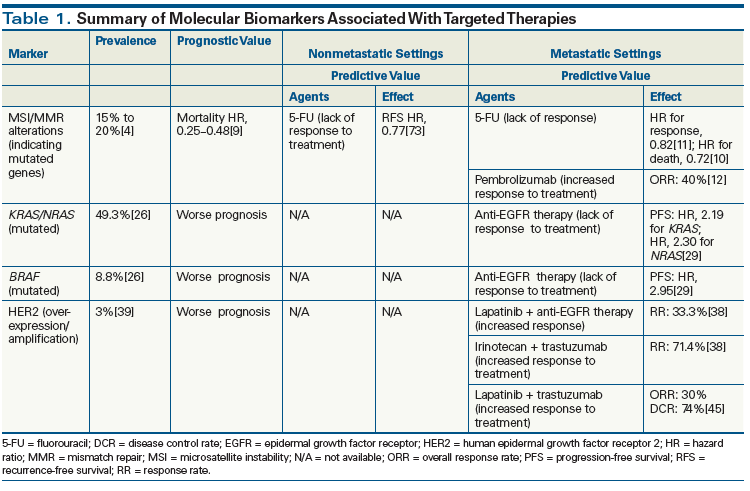
Most studies indicate that MSI-H status in colorectal cancer is prognostic of a better clinical outcome compared with MSI-L/MSS disease.[9] In one study, colorectal cancer–specific mortality hazard ratios (HRs) for MSI-H vs MSI-L/MSS were 0.48 (95% CI, 0.27–0.87; P = .02) and 0.25 (95% CI, 0.12–0.52; P < .001) for BRAF-mutated and wild-type disease, respectively.[9] Other studies suggest that the favorable prognosis of MSI-H may be limited to patients without BRAF mutations.[9] In the adjuvant setting, MSI-H status is a predictive marker for lack of response to fluorouracil (5-FU)-based chemotherapy compared with MSS disease.[10] In fact, in one study of 570 patients, there was a statistical trend towards worse disease-free survival and OS in patients with MSI-H disease treated with adjuvant 5-FU-based chemotherapy compared with patients who did not receive 5-FU.[10] Consequently, the presence of MSI-H is often used to identify patients with stage II disease who should not be offered adjuvant chemotherapy. It is unknown whether or not patients with MSI-H stage II disease and BRAF mutations benefit from adjuvant chemotherapy. The role of MSI status in decision making regarding chemotherapy for stage III colorectal cancer is less clear.
MSI-H does not appear to have the same predictive value for chemotherapy response in the metastatic setting. In one study, the HR for response was 0.82 (95% CI, 0.65–1.03; P = .09), reflecting a trend towards a better treatment outcome than that of patients with MSI-L/MSS tumors but failing to reach statistical significance.[11] However, recent data suggest that patients with MSI-H metastatic disease respond preferentially to immune checkpoint inhibition with the programmed death 1 (PD-1) inhibitor pembrolizumab. In a recent phase II study of 41 heavily pretreated patients with progressive metastatic carcinoma, the objective response rate (ORR) based on immune-related response criteria was 40% (4 of 10 patients) and the progression-free survival (PFS) rate was 78% (7 of 9 patients) in the patients with MSI-H colorectal cancer. In the patients with MMR-proficient tumors (ie, MSS tumors, MSI-L tumors, and tumors with intact MMR proteins), the corresponding response rates were 0% (0 of 8 patients) and 11% (2 of 18 patients), respectively.[12,13] Notably, the microenvironment in MMRD tumors (those with MSI; ie, large numbers of genomic mutations, particularly in areas of repetitive DNA sequences) has been found to be highly immunogenic. In addition to a microenvironment characterized by dense infiltration with immune cells and T helper type 1–associated cytokines,[14,15] recent studies have shown that MMRD tumors strongly express PD-1, programmed death ligand 1 (PD-L1), cytotoxic T-lymphocyte–associated antigen 4, the LAG3 protein, and indoleamine 2,3-dioxygenase; these findings suggest that the immune-active microenvironment of MMRD tumors is counterbalanced by immune inhibitory signals resisting tumor cell apoptosis, and that these signals represent targets for immune checkpoint inhibitor therapy.[14,15] Larger, prospective studies are needed in order to confirm this clinical observation. Additional studies are also needed to clarify the role of immune checkpoint inhibition in both untreated metastatic disease and in high-risk stage II and III colorectal cancer with MSI-H.
The greater susceptibility to immune checkpoint inhibition in tumors with MMRD has been hypothesized to stem from the high numbers of mutation-associated tumor neoantigens arising from the many somatic mutations occurring in tumors with MSI. In fact, Le et al found that MMRD tumors carried a mean of 1,782 somatic mutations per tumor and an average of 578 potential mutation-associated neoantigens, whereas tumor samples from patients with MMR-proficient colorectal cancers had a mean of 73 mutations per tumor and an average of 21 mutation-associated neoantigens.[13] Recognition of mutation-associated neoantigens is an important component of the endogenous antitumor immune response; this has been supported by research showing that higher mutational load in patients with bladder cancer, melanoma, and lung cancer was correlated to a better response to treatment with various immune checkpoint inhibitors.[14,16-18] Notably, a study from Memorial Sloan Kettering Cancer Center showed that using next-generation sequencing panels to evaluate mutational load in colorectal cancer provided a reliable surrogate for MSI testing to identify patients who could potentially respond to treatment with immune checkpoint inhibitors.[19] Indeed, high-throughput sequencing technologies could also be used to identify oncogenic drivers and therapeutic targets in tumor tissue, as well as in the evaluation of the immunogenicity of the tumor microenvironment.
Immunoscore, an Emerging Prognostic Marker
As evidence accumulates regarding the importance of the tumor microenvironment in disease progression, disease recurrence, and the potential response to immune modulatory agents, Immunoscore, a scoring system to evaluate in situ immune cell infiltration in colorectal cancer, is emerging as an important prognostic marker. The Immunoscore is based on quantification of cytotoxic and memory T cells in the core of the tumor and in the tumor’s invasive margin, and has been shown to be highly associated with patient survival; further, the naturally infiltrating T cells have demonstrated susceptibility to enhancement by immunotherapies.[20]
Immunoscore provides scores on a scale of I0 to I4 to stratify patients based on the density and location of immune cells in primary tumors; patients with low densities of CD3 and CD8 are scored as I0, whereas patients with high densities of these markers are scored as I4. Interestingly, a recent study indicates that although I4 tumors are overrepresented in patients with MSI compared with MSS patients, not all tumors with MSI have intrinsically more immunogenicity, and a subgroup of MSS patients also have immunogenic tumors.[20] When survival outcomes are compared, MSI and MSS patients with a low Immunoscore had a higher risk of relapse (HR, 2.4; CI, 1.1–5.24; P = .023), shorter disease-specific survival (HR, 3.4; CI, 2.3–5.05; P = 6.9E-11), and shorter OS (for MSI patients, HR, 1.8; CI, 1.04–3.11; P = .033; and for MSS patients, HR, 2.43; CI, 1.81–3.26; P = 1E-9) compared with MSI and MSS patients with a high Immunoscore, suggesting its important prognostic role. Further, patients with a high Immunoscore also showed higher expression of PD-1 and PD-L1 vs those with a low Immunoscore, independent of MSI status.[20] In addition to the correlation of a patient’s Immunoscore with survival and immune inhibition, a high Immunoscore is also associated with a decreased propensity to develop distant metastasis. In a recent study of 838 patients with colorectal cancer, when patients were divided into two cohorts-those with no metastasis (M0) vs metastasis (M1), no difference was found in terms of chromosomal instability or key cancer-associated gene mutations.[21] When patients were stratified by Immunoscore into I0 (low) to I4 (high) cohorts, I0 tumors were significantly overrepresented in the M1 vs the M0 cohort, and low Immunoscore was associated with a shorter OS compared with I4. The authors found that, even in cases of stage IV colorectal cancer, an Immunoscore of I4 identified the patients with the longest OS (with an OS rate of 65% at 5 years in the I4 group).
The concept of quantitating lymphocyte infiltration has existed in the research setting for several years, and well-controlled international multicenter consortium studies have brought the lymphocyte count closer to use as a clinical marker.[22] In a study of more than 1,300 patients with stages I, II, or III colorectal cancer, time to recurrence was shorter in patients with a low vs high Immunoscore (HR, 0.35; 95% CI, 0.23–0.52; P < .0001 in the training set; and HR, 0.54; 95% CI, 0.34–0.84; P = .006 in the validation set).[23] Although these results are very promising, validation from an independent group, standardization of the immunoscoring methods used in clinical trials, and integration of immunoscoring with other prognostic factors in colorectal cancer are needed before the marker can be incorporated into clinical practice.
RAS/RAF
The epidermal growth factor receptor (EGFR) is a cell surface receptor that, once activated, promotes cell division and survival. The mechanism of message signaling includes the constitutive activation of the mitogen-activated protein kinase (MAPK) pathway, which is comprised of the RAS-RAF-MEK-ERK signaling cascade and is deregulated in more than half of colorectal tumors. The most important downstream molecules include RAS and BRAF.[24] The RAS family includes the proto-oncogenes KRAS, NRAS, and HRAS, as well as many other members; mutation in these molecules can independently promote cancer cell division, survival, and metastasis.[25] The frequency of mutations is 42.4% for KRAS codons 12 and 13; 6.9% for NRAS and other RAS mutations; and 8.8% for BRAF mutations.[26]
Generally, patients whose tumors contain RAS/RAF mutations have a worse prognosis than those with wild-type tumors.[27,28] However, Roth et al demonstrated no prognostic value for KRAS mutation status in stage II and stage III colorectal cancer.[28] Mutations involving RAS have been proven to be negative predictive markers for response to anti-EGFR therapy. Therkildsen et al demonstrated worse outcomes when anti-EGFR agents were given to patients with RAS-mutated metastatic colorectal cancer.[29] For KRAS exon 3 and exon 4 mutations, the HR was 2.19 (95% CI, 1.43–3.35) for PFS and 1.78 (95% CI, 1.29–2.44) for OS. For NRAS mutation, the HR was 2.30 (95% CI, 1.30–4.07) for PFS and 1.85 (95% CI, 1.23–2.78) for OS.[29] Patients with BRAF-mutated metastatic colorectal cancer had shorter PFS (HR, 2.95; 95% CI, 1.89–4.61) and OS (HR, 2.52; 95% CI, 1.39–4.56). On the other hand, the predictive value of BRAF mutation status is still being investigated. Pietrantonio et al demonstrated the results of using anti-EGFR therapy in 463 patients with BRAF-mutated metastatic colorectal cancer.[30] The addition of an anti-EGFR agent did not have a significant impact on PFS (HR, 0.88; 95% CI, 0.67–1.14; P = .33), OS (HR, 0.91; 95% CI, 0.62–1.34; P = .63), and ORR (relative risk, 1.31; 95% CI, 0.83–2.08; P = .25) compared with control regimens. This can be explained by the overall worse prognosis associated with BRAF mutations. Multiple trials are underway to address the predictive value of BRAF mutation status in decision making regarding the use of anti-EGFR therapies. Early clinical trials combining BRAF inhibitors with EGFR-targeted therapies (ClinicalTrials.gov identifiers: NCT02928224, NCT01787500) have shown promise.[31]
Currently, most guidelines recommend testing for RAS/RAF mutations to inform treatment decisions in the metastatic setting only. A variety of commercial PCR assays and gene testing panels are available for mutation testing. Based on prospective-retrospective analysis of the PRIME trial of RAS mutations in colorectal cancer and the correlative study evaluating mutations at different loci of this gene, the recommendations have been expanded to include testing for mutations in other codons besides KRAS codons 12 and 13 in exon 2, such as KRAS codons on exons 3 and 4, as well as NRAS and BRAF.[25] Santini et al demonstrated the high concordance of KRAS status between the primary colorectal tumors and related metastatic sites; among 99 patients, the disconcordance rate was only 4%.[32] Fedyanin et al demonstrated a higher disconcordance rate: 26.3% for KRAS and 5.2% for NRAS.[33] Since anti-EGFR therapy is recommended in the metastatic setting only, there is no indication for RAS or BRAF testing in the nonmetastatic setting. Repeat testing may be warranted in selected cases of isolated lesion progression. There is still no consensus on whether this approach should be adopted as standard practice.
In addition to primary resistance to anti-EGFR treatment conferred by de novo RAS mutations, acquired RAS mutations detected in circulating tumor DNA (ctDNA) have been reported in patients who developed resistance to treatment. These variants are hypothesized to have arisen from either an acquired mutation of KRAS or from a small preexisting subclone of cancer cells that expanded under the selection pressure exerted by the targeted treatment. The first such report, in a study of 10 patients, was published in 2012; it showed that KRAS mutations in codon 61 (Q61H) and codon 13 (G13D) could be detected in ctDNA, and accounted for resistance to cetuximab or panitumumab.[34] In a recent study by Siravegna et al, among 16 patients who initially responded to anti-EGFR therapy but later became resistant to treatment, 11 had KRAS-mutated alleles detected in blood samples obtained at the point of disease progression.[35] Notably, the authors found that suspending anti-EGFR treatment led to a decreased level of mutant KRAS allele in ctDNA; however, rechallenging with anti-EGFR therapy at this time produced a significant tumor response, after which the KRAS allele began to reappear. Monitoring of KRAS allele levels could therefore be useful in decision making regarding intermittent administration of anti-EGFR therapy, which may prevent the accumulation of tumor clones.[35]
A key challenge in clinical adoption of ctDNA evaluation is the need for a highly sensitive test. Current methodologies include digital PCR-based BEAMing (Beads, Emulsion, Amplification, Magnetics) tests and their variations. The reported concordance rate achieved, when comparing the tumor results with ctDNA from pretreatment samples, has ranged from approximately 78% to 92%.[36] In a study evaluating ctDNA in 206 patients with colorectal cancer, the sensitivity of detection was 87.2% and the concordance rate of KRAS mutational status prior to treatment in the tumor and plasma was 88%; these findings are similar to the results of other recently reported but smaller studies.[37] Further, in a study by Siravegna et al of ctDNA alterations, 8 of the 100 tumors studied harbored mutations that were not detected in the matched tissue, suggesting that evaluating blood samples more comprehensively captured intrapatient tumor heterogeneity.[35] With the advancement of sequencing technologies and the higher sensitivities achieved with these approaches, the role of liquid biopsy in oncology will continue to expand.
HER2
EGFR is a transmembrane receptor tyrosine kinase in the ERBB family, which also includes four receptor tyrosine kinases structurally related to EGFR: ERBB1 (HER1), ERBB2 (HER2/neu), ERBB3 (HER3), and ERBB4 (HER4). With the exception of HER2/neu, the ERBB family of proteins can bind to multiple ligands.[38] EGFR functions by activating several different signaling pathways, including RAS-MAPK and PI3K-AKT.[39] Overexpression of the HER2 protein is found in approximately 3% of colorectal cancer cases, with HER2 amplification being the most common mechanism causing overexpression.[39] Amplification of HER2 has been reported in 13% to 36% of patients with cetuximab-resistant colorectal cancer[38,39] and can be detected in tumor samples using either IHC or fluorescence in situ hybridization (FISH).[40]
The use of HER2 testing in the management of colorectal cancer has not yet been incorporated into routine clinical practice. Because HER2-amplified tumors are found more frequently in patients with resistance to anti-EGFR agents, some studies have recommended HER2 testing prior to initiation of anti-EGFR therapy.[41] Extensive HER2 amplification in KRAS wild-type colorectal cancer resistant to anti-EGFR therapies was found to be associated with worse outcomes (HR for PFS, 3.65, P = .0026; HR for OS, 5.05, P = .0002).[39] As HER2 amplification becomes a recognized mechanism of resistance to anti-EGFR agents, there has been increasing investigation of various strategies employing anti-HER2 therapy in colorectal cancer management. In a small study, the combination of lapatinib and cetuximab yielded a partial response in 2 of 6 patients with colorectal cancer, and disease control in 5 of 6 patients. In the same study, treatment with irinotecan and trastuzumab produced a partial response in 5 of 7 patients with metastatic colorectal cancer and HER2 protein overexpression.[38]
In preclinical studies of colorectal cancer, monotherapy with either an anti-HER2 antibody or a small-molecule tyrosine kinase inhibitor of HER2 was mostly ineffective, likely due to upregulation and reactivation of HER3. These findings could potentially account for why some early clinical studies failed to show efficacy of HER2-targeted therapy in colorectal cancer.[42,43] However, in preclinical models, a combination of the two drug groups effectively suppressed HER3 phosphorylation and led to a sustained treatment response.[44] In fact, the exciting results of HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification), a multicenter, proof-of-concept, open-label, phase II trial, showed that the combination of lapatinib plus trastuzumab, an approved treatment for HER2-positive breast cancer and gastric cancer, is effective in heavily pretreated patients who are refractory to anti-EGFR therapy, in the absence of any chemotherapy backbone.[45] In this trial, 914 KRAS wild-type patients were screened for HER2 positivity; 5% of the tumors were shown to be HER2 positive. Among the 27 patients eligible for response evaluation, 8 (30%) patients achieved an objective response, with 1 patient achieving a complete response and 7 patients achieving partial responses; 12 patients (44%) achieved stable disease. The trial also brought to light emerging colorectal cancer–specific diagnostic criteria that define HER2 positivity by use of IHC and FISH (HERACLES diagnostic criteria). Interestingly, post-hoc analysis of this study showed that a higher level of HER2 gene activity is associated with better treatment outcomes, in that responses were significantly more common in tumors with HER2 gene copy numbers above rather than below the threshold.[45] Overall, the results of the HERACLES trial confirm that HER2 is an effective therapeutic target and the evaluation of HER2 status in patients with colorectal cancer will likely be incorporated into clinical practice in the near future.
TS
The fluoropyrimidine 5-FU is the cornerstone of combination chemotherapy used in the treatment of colorectal cancer.[46] 5-FU is used in both the adjuvant and metastatic settings, as a single agent and in combinations, in IV and oral forms; essentially all patients with colorectal cancer will receive this treatment. Clinicians have more than 60 years of experience using 5-FU for the treatment of colorectal cancer; this agent has been extensively studied in order to better understand the optimal dose and schedule, as well as the ideal treatment population. 5-FU exerts its anticancer effects by inhibiting thymidylate synthase (TS) and incorporating its metabolites fluorouridine triphosphate (FUTP) and fluorodeoxyuridine triphosphate (FdUTP) into RNA and DNA, respectively, making TS a potential predictive biomarker in colorectal cancer.[47-50]
TS is an enzyme that catalyzes the reductive methylation of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), which is subsequently phosphorylated to thymidine triphosphate for de novo DNA biosynthesis and DNA repair.[48] TS is an important target for many chemotherapy agents, including 5-FU, 5-fluorodeoxyuridine, raltitrexed, pemetrexed, and others.[48] TS gene amplification, increased TS protein stability, and translational derepression leads to increased TS protein levels and resistance to TS-targeting drugs.[48]
Biomarkers such as TS that are measured using IHC require stringent control to minimize interpersonal variability of data interpretation and rigorous scrutiny to reduce inter-batch variability of the antibody. In addition, it is important to consider the antibody used for testing and the threshold that is set for a positive vs negative result. Moreover, preanalytic tissue controls are needed to optimize the results of TS testing, since the presence of ischemia alters TS expression[51] and has a significant impact on TS testing results. None of the studies we cite in this article control for the ischemia time of the tissue being tested.
A number of clinical studies of the potential association between TS expression and the outcome of treatment with 5-FU have generated conflicting results. In a meta-analysis summarizing data from 1,112 patients enrolled in 24 studies, the relative ratio for ORR was 2.20 (95% CI, 1.82–2.66; P = .000), and the effect was more prominent when TS expression level was measured from the metastatic lesions.[52] Due to the lack of data from a large prospective randomized controlled trial to guide decision making regarding TS testing, most clinics do not test TS levels routinely. Using modern testing standards for TS, 16% of all patients with colorectal cancer (922 of 5,711) had high levels of TS,[53] suggesting that this cohort would be less likely to benefit from treatment with 5-FU. If validated, TS testing could potentially greatly improve overall treatment outcomes for patients with colorectal cancer.
High TS expression is a negative prognostic marker for colorectal cancer and indicative of increased cellular proliferation, tumor invasiveness, and metastatic capacity.[48] Patients with low levels of TS expression had a longer median OS compared with patients with high TS levels (102 months vs 69.8 months; HR, 0.61; 95% CI, 0.55–0.68; P < .0001).[54] However, high TS expression was not significantly associated with any change in OS (HR, 0.47; 95% CI, 0.31–0.94; P = .16).[54,55]
Low levels of TS expression have shown slight correlation with improved response rate and survival in patients treated with FOLFOX (leucovorin, 5-FU, and oxaliplatin); the median OS was longer for those with low TS expression (36.0 months vs 14.8 months; HR, 0.37; 95% CI, 0.076–0.80; log-rank P = .022).[54] However, after controlling for covariates in a Cox model, the association of OS with TS expression levels was no longer significant (HR, 0.47; 95% CI, 0.31–0.94; P = .16); however, the investigators found an association between Eastern Cooperative Oncology Group performance status and survival (HR, 0.35; 95% CI, 0.28–0.48; P = .006). There was a slight trend in the same direction for median time to treatment failure in the low TS expression group vs the high TS expression group (12.3 months vs 11.7 months; HR, 0.82; 95% CI, 0.33–2.0; log-rank P = .18), but this did not reach statistical significance. The ORR to any therapy in the patients with low TS expression level was 86% compared with 58% in the high TS expression level group (relative risk, 0.68; 95% CI, 0.41–1.1; Fisher’s exact P = .10).[54]
TS is not currently an established marker to be evaluated in all patients with colorectal cancer. Past studies attempting to evaluate its role as predictor of response to chemotherapy have yielded conflicting results. However, the technology to measure TS has evolved; notably, IHC has been replaced with reverse transcriptase PCR, which has been shown to result in TS expression testing with a better predictive value.[52] The frequency of increased TS expression compels us to clarify the role that this biomarker plays in a fundamental agent such as 5-FU in colorectal cancer. We are currently performing a prospective trial to determine the value of TS measurement in outcomes for patients with metastatic colorectal cancer.
UGT1A1
Irinotecan is one of several important chemotherapeutic agents used in the treatment of advanced colorectal cancer and multiple other tumor types, including pancreatic, esophageal, and lung cancers. In the treatment of colorectal cancer, irinotecan is regularly used as a single agent, or in 5-FU–based regimens such as FOLFIRI (leucovorin, 5-FU, and irinotecan) or FOLFOXIRI (leucovorin, 5-FU, oxaliplatin, and irinotecan). Irinotecan exerts its cytotoxic activity after being converted into the active metabolite SN-38, which is responsible for both the efficacy and the major toxicities of irinotecan (principally neutropenia and diarrhea). The main method of inactivation and clearance of SN-38 is via glucuronidation in the liver by UDP-glucuronosyltransferases (UGT1A1), followed by excretion of this water-soluble catabolite in the bile or urine.[56] There are other glucuronosyltransferases (UGT1A7 and UGT1A) and cytochrome P450 family 3 (CYP3A) enzymes that can inactivate SN-38, but the well-characterized gene variants described below are the focus of clinical interest in colorectal cancer.
More than 100 variants in the UGT1A1 gene have been found, with varying effects on the expression of the protein.[57] The gene is located on chromosome 2q37, and alterations have been demonstrated in the promoter region and the five exons. The most common alterations are found in the promoter region, where there are variable numbers of thymine-adenine (TA) repeats in the TATA box.[58] The wild-type allele (UGT1A1*1) includes six repeats (and so is often referred to as UGT1A1*6); the most common variant includes seven repeats (UGT1A1*28, also known as UGT1A1*7), and is associated with an approximately 70% reduction in enzymatic activity when the gene is homozygous. In Caucasians, approximately 30% to 50% are homozygous wild-type (the UGT1A1*1/*1 genotype), 40% to 50% are heterozygous (UGT1A1*1/*28), and 8% to 20% are homozygous for *28/*28 (also known as the UGT1A1*7/*7 genotype). In the United States, the frequency of the UGT1A1*28 allele varies among ethnicities, the highest being in African Americans (occurring with an allele frequency of 0.426), with substantive occurrence in Caucasians (0.387 allele frequency) and Asians (0.16 allele frequency).[59] Homozygous UGT1A1*28 accounts for most cases of Gilbert syndrome and is associated with mild elevation of bilirubin levels, but individuals with this mutation are otherwise asymptomatic. The complete loss of glucuronidation can result in Crigler-Najjar syndrome, which can cause death in neonates from kernicterus (encephalopathy resulting from the accumulation of bilirubin in the brain and nerve tissues) or neurologic impairment in infants who survive, unless bilirubin levels are brought under control.
In contrast, other variants are more common in Asian populations, the most frequent being UGT1A1*6, which also has about 70% reduction in enzymatic activity, and UGT1A1*27, a mutation characterized by almost complete loss of enzymatic activity. Both variants are homozygous in about 3% of Asians and result from single nucleotide polymorphisms (SNPs) in exon 1. Variants UGT1A1*60 and UGT1A1*93 result from alterations in the phenobarbital response enhancer module 5 UGT1A1 and are homozygous in approximately 10% of Caucasians.[58]
Some variants may also be associated with lower levels of bilirubin and decreased toxicity from irinotecan, which is linked to increased glucuronidation. For example, in the 3-flanking region of UGT1, a variant G allele (rs11563250G) has been noted in about 12% of the population. Patients with this marker may tolerate treatment with higher levels of irinotecan.[60]
As noted, the most common alleles are UGT1A1*1 and UGT1A1*28. Standard testing for gene polymorphism by PCR analysis assesses the presence of these two alleles, but this approach can miss other variants, such as the UGT1A1*27 and UGT1A1*6 variants that occur with greater frequency in Asians. The measurement of total bilirubin and unconjugated bilirubin has also been shown to be associated with UGT1A1*28/*28; Yu et al found that using combination cutoff values of 0.8 mg/dL for total bilirubin levels and 0.2 mg/dL for unconjugated bilirubin levels was predictive of the presence of this genotype.[61]
KEY POINTS
- Molecular profiling has led to improvements in colorectal cancer treatment strategies.
- Testing for KRAS, NRAS, and BRAF is an important tool to guide anti–epidermal growth factor receptor therapy in metastatic colorectal cancer.
- Mismatch repair deficiency and microsatellite instability are important markers for the use of immunotherapy in colorectal cancer.
- Many molecular markers are being investigated to improve treatment strategy and treatment dosing in the management of colorectal cancer.
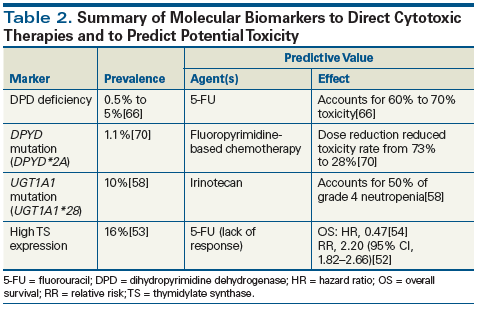
Overall, approximately 10% of Caucasian patients are homozygous for the UGT1A1*28 variant, the presence of which has been associated with increased risk of irinotecan-induced neutropenia and diarrhea.[58,62] The need for testing appears to depend on the planned dose of irinotecan to be administered. Planned doses of irinotecan exceeding 250 mg/m2 appear to pose a greater risk of these treatment toxicities in colorectal cancer patients whose tumors carry the UGT1A1*28 variant.[63] These findings resulted in a US Food and Drug Administration (FDA) warning that patients who are homozygous for the UGT1A1*28 allele are at increased risk for neutropenia. In a study of 66 patients receiving irinotecan at a dosage of 350 mg/m2 every 3 weeks, grade 4 neutropenia was found in 50% of patients with the UGT1A1*28/*28 variant, 12.5% of UGT1A1*1/*28 heterozygotes, and 0% in those homozygous for wild-type UGT1A1*1/*1.[58] This led to recommendations (by the Pharmacogenetics Working Group of the Royal Dutch Association for the Advancement of Pharmacy) that patients homozygous for UGT1A1*28/*28 have the irinotecan dose reduced by 30% when treatment doses greater than 250 mg/m2 are planned.[63] The assessment of UGT1A1 status has been found to be cost-effective, given the increased costs associated with treatment-related toxicities in these patients.[56]
There have been some indications that the response to treatment may also be improved in patients with the UGT1A1*28/*28 variant, but this has not been seen in all studies.[64] For example, in the study of 120 patients by Yu and colleagues, the presence of UGT1A1*28/*28 or elevated levels of total bilirubin and unconjugated bilirubin were actually associated with a lower response rate.[61] This could not be explained by decreases in doses associated with toxicity.
DPD
The cytotoxicity of 5-FU occurs via three principal mechanisms: 1) interference with the synthesis of thymidine monophosphate by the binding of fluorodeoxyuridine monophosphate (FdUMP) to TS; 2) incorporation of FdUTP into DNA, thereby interfering with DNA synthesis; and 3) incorporation of FUTP into RNA, leading to faulty translation of RNA and blocking the synthesis of multiple forms of RNA (ie, messenger, ribosomal, transfer, and small messenger RNAs).[65] The metabolic breakdown of 5-FU is primarily through the action of dihydropyrimidine dehydrogenase (DPD), which catabolizes 5-FU to 5-fluoro-5,6-dihydrouracil. DPYD, the gene coding for DPD, is present on chromosome 1, with 23 exons in a single copy. More than 80% of an administered dose of 5-FU is catabolized via this pathway, although some 5-FU is excreted unchanged in the urine.[49]
Loss of DPD activity has been well characterized as an autosomal recessive trait, with a prevalence of 0.5% and 5% for total and partial deficiencies, respectively.[66] When receiving a standard dose of 5-FU, individuals carrying certain variants of DPYD are at significantly greater risk for severe and potentially lethal adverse events (grade 3 or higher). Early studies indicated that almost one-third of the severe toxicities reported with 5-FU could be attributed to partial or total alteration in DPD activity.[49,66] More recent analyses have attributed at least 60% to 70% of cases of 5-FU toxicity to decreased levels of DPD. Among all variants studied, DPYD*2A (c.1905+1G>A; rs3918290) is the most common allele associated with loss of DPD activity. Many other alleles have also been associated with DPD loss and 5-FU toxicity, including DPYD c.2846A>T (D949V, rs67376798), c.1679T>G (I560S, DPYD*13, rs55886062), c.1236G>A/HapB3 (rs56038477/ rs75017182), and c.1601G>A (S534N, DPYD*4, rs1801158), but the results have been somewhat variable.[67]For example, a meta-analysis found that the variant c.1601G>A was not significantly associated with toxicity; a recent large study of stage III colon cancer showed that the variant c.1236G>A/HapB3 also was not associated with toxicity.[68] Work is ongoing to evaluate the individual genetic variant changes associated with 5-FU toxicity. It should be noted that other genetic alterations might also be associated with 5-FU toxicity, such as the loss of TYMS, which codes for TS.[69]
Most of these analyses were carried out using SNPs, and a variety of SNP genotyping assays are commercially available. Some pharmaceutical companies are only evaluating DPYD*2A by SNP analysis; others are focusing on four SNPs or conducting full nucleotide sequence analysis. The former approach of evaluating DPYD*2A alone has been found to be clinically practical and to decrease toxicity and its associated costs.[70] In a large clinical trial of more than 2,000 patients prospectively genotyped for DPYD*2A, variant carriers were treated with dose reductions of at least 50%, while wild-type patients were treated with standard dose.[70] The rate of grade 3 or higher toxicity was reduced from 73% to 28% in the variant carriers, similar to the rate of severe toxicity observed in noncarriers of *2A. Furthermore, it was estimated that average total treatment cost per patient was lower for screening than for nonscreening, outweighing the costs associated with screening. In contrast, the recent FDA approval of uridine triacetate has provided an emergency treatment option for patients who experience severe or life-threatening toxicity while receiving 5-FU, but administration of this antidote must begin within 96 hours, and a 5-day therapy course costs about $80,000. Though exciting, this large clinical trial considered only DPYD*2A variants, which only accounts for 25% of DPD-deficient patients, while more recently identified DPYD polymorphisms previously mentioned were not included; further, only 18 carriers received reduced-dose therapy. Nevertheless, the study shows that frontline genotyping is efficacious and cost-effective, and further large-scale prospective studies will bring the test closer to clinical practice.
Ongoing trials continue to employ a variety of assays. For example, a study in the Netherlands (ClinicalTrials.gov identifier: NCT02324452) is using SNPs to assess four different mutations to alter treatment, as well as cost and efficacy. In addition to SNP genotyping, the investigators are measuring the clearance of [2-13C]–labeled uracil in the breath, the serum level of uracil after an oral dose, and enzymatic DPD activity in peripheral blood mononuclear cells. Alterations in DPD activity are clearly associated with severe toxicity from 5-FU and other fluoropyrimidines in a relatively small number of patients. A variety of mutations can cause such a deficiency, and the optimal method for evaluation remains an area of ongoing research. A major challenge with genotyping for DPYD is that patients who do not carry any DPYD variant can still develop severe toxicity, while some patients carrying a DPYD variant do not develop any toxicity.[71] Some investigators suggest that at a minimum, genotyping of the DPYD*2A variant using SNP assays is worthwhile, but more sophisticated testing may predict greater numbers of patients likely to benefit from decreasing the treatment dosage of 5-FU.
The Consensus Molecular Subtypes of Colorectal Cancer
With the advancement of molecular technologies and the increasing capability of interrogating tumor tissue for various alterations, molecular subtyping of colorectal tumors has been attempted by multiple groups, generating a large amount of data; however, the results shared limited similarities. In order to resolve the inconsistencies and to establish a subtyping standard that can potentially be used to predict patient outcome and to tailor treatment, the international Colorectal Cancer Subtyping Consortium was formed and evaluated six independently developed colorectal cancer subtyping algorithms. These resultant CMS1–4 designations provided insight into the biological understanding of each subtype, as well as specific associated clinical and prognostic factors.[72]
CMS1, or the MSI immune group, is consistent with the established MSI phenotype.[73] It comprises 14% of the patient population; occurs more frequently in older, female patients; commonly presents as MSI and CIMP; and is associated with high rates of BRAF mutation and high levels of immune activation. CMS2, the canonical subgroup, is the most common subtype and comprises 37% of cases. These tumors are characterized by chromosomal instability, with marked activation of the WNT and MYC pathways, and high rates of TP53 mutation. These tumors are associated with the longest OS times and longest survival after relapse, and are found in more than half of tumors of the left colon and the rectum. CMS3, or the metabolic subtype, comprises 13% of tumors; similar to KRAS activating mutations, the tumor cells are enriched for multiple metabolism signatures. These tumors are associated with intermediate OS times compared with the other three subtypes. The CMS4 subtype, or mesenchymal tumors, comprise 23% of cases and exhibit prominent mesenchymal/transforming growth factor-β activation, stromal invasion, and angiogenesis. These patients are diagnosed at a younger age and have the worst OS outcomes of the four subtypes. Notably, none of the molecular subtypes were defined by an individual event, and no genetic aberration was limited to one particular subtype. The reliance on an integrative analysis of alterations obtained from multiple platforms emphasizes the molecular complexity of the subtypes. These consensus subtypes defined from various databases via a uniform algorithm established an important paradigm for a collaborative, community-based cancer subtyping strategy that can be adopted in the management of a variety of cancer types to utilize the large amount of emerging data and to resolve inconsistencies; however, further investigation is needed to clarify how to utilize these subtypes in the clinic to guide patient therapy.
Conclusion
Advances in the technology of tumor testing have enhanced our understanding of the molecular and genetic basis of a variety of cancer types, including colorectal cancer. This expanding body of knowledge has informed clinical trials that have led to an increased number of potential prognostic and predictive markers relevant to the management of patients with colorectal cancer. Personalized medicine, with treatment tailored based on the tumor’s molecular and genetic signature, remains a goal in the treatment of colorectal cancer. In this article we have reviewed some of the common markers used to guide therapy; however, for some of these putative markers there may be insufficient data or statistical power to establish their ability to affect treatment decisions. In patients with colorectal cancer, the use of markers that predict resistance to targeted therapies such as anti-EGFR has been proven to be cost-effective as well as clinically critical, and therefore has been incorporated into clinical guidelines. Similarly, MSI testing has been used to guide therapy in the setting of early disease and more recently to guide immunotherapy in patients with metastatic colorectal cancer. However, the use of other markers that predict response to targeted therapy is still lagging behind, perhaps due to the limited prevalence of these markers in colorectal cancer or the lack of strong prospective evidence to support their use. Hence, we recommend MSI testing (preferably IHC testing for MMR proteins) for all patients. We also recommend testing for mutations of KRAS, NRAS, and BRAF (any of the available approved methods) for patients with metastatic colorectal cancer only. Testing for HER2 gene amplification and HER2 protein overexpression should be limited to patients with metastatic colorectal cancer for whom standard treatment options have failed (Table 1).
The use of markers to predict the toxicity of treatment with cytotoxic agents is not yet widespread due to the limited prevalence of relevant markers; the lack of prospective evidence supporting their incorporation into routine patient care; and current limitations in testing sensitivity, specificity, and interpretation. Although these markers can guide individual therapy in selected patients (Table 2), at the general population level, collaborative work is needed in order to identify more markers, prospectively validate their prognostic and predictive value, and evaluate the cost-effectiveness of testing each marker. Hence, we recommend UGT1A1*28 testing in patients for whom treatment with irinotecan is planned at a dose higher than 250 mg/m2. On the other hand, testing for mutations of TS and DPYD can be considered in selected patients with colorectal cancer.
Finally, although this article has focused on reviewing the impact of single genetic markers associated with the response to single-treatment agents, it is possible that a multimarker modality may be required to achieve the promise of true personalized medicine in colorectal cancer. However, this approach inevitably leads to hypersegmentation of patient cohorts, a clinical challenge that can only be addressed by broad collaborative projects with established standards and requirements for biomarker testing.
Acknowledgment: The authors thank David Spetzler, PhD, MS, MBA, President and Chief Scientific Officer, Caris Life Sciences; and Joanne Xiu, PhD, Director of Medical Affairs, Caris Life Sciences, for their contributions to the editorial development of this article.
Financial Disclosure:Dr. Marshall has relationships with the following companies, for which he has served as an advisor, consultant, researcher, and speaker: Amgen, Bayer, Caris Life Sciences, Celgene, Genentech, and Taiho. Dr. Shields has received funding for research and travel from Caris Life Sciences. The other authors have no significant interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809-24.
3. Setaffy L, Langner C. Microsatellite instability in colorectal cancer: clinicopathological significance. Pol J Pathol. 2015;66:203-18.
4. Boland CR, Koi M, Chang DK, Carethers JM. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Fam Cancer. 2008;7:41-52.
5. Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153-62.
6. Zhang CM, Lv JF, Gong L, et al. Role of deficient mismatch repair in the personalized management of colorectal cancer. Int J Environ Res Public Health. 2016;13:E892.
7. Cicek MS, Lindor NM, Gallinger S, et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn. 2011;13:271-81.
8. Loughrey MB, Waring PM, Tan A, et al. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam Cancer. 2007;6:301-10.
9. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151-6.
10. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247-57.
11. Des Guetz G, Uzzan B, Nicolas P, et al. Microsatellite instability does not predict the efficacy of chemotherapy in metastatic colorectal cancer. A systematic review and meta-analysis. Anticancer Res. 2009;29:1615-20.
12. Yiu AJ, Yiu CY. Biomarkers in colorectal cancer. Anticancer Res. 2016;36:1093-102.
13. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-20.
14. Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417-22.
15. Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51.
16. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909-20.
17. Rizvi NA, Hellman MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-8.
18. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189-99.
19. Stadler ZK, Battaglin F, Middha S, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. 2016;34:2141-7.
20. Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698-711.
21. Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26.
22. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199-209.
23. Galon J, Mlecnik B, Marliot F, et al. Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: results of a worldwide consortium-based analysis of 1,336 patients. J Clin Oncol. 2016;34(suppl):abstr 3500.
24. Kassouf E, Tabchi S, Tehfe M. Anti-EGFR therapy for metastatic colorectal cancer in the era of extended RAS gene mutational analysis. BioDrugs. 2016;30:95-104.
25. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-34.
26. Vaughn CP, Zobell SD, Furtado LV, et al. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50:307-12.
27. Rui Y, Wang C, Zhou Z, et al. K-Ras mutation and prognosis of colorectal cancer: a meta-analysis. Hepatogastroenterology. 2015;62:19-24.
28. Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466-74.
29. Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol. 2014;53:852-64.
30. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587-94.
31. Cohen R, Cervera P, Svrcek M, et al. BRAF-mutated colorectal cancer: what is the optimal strategy for treatment? Curr Treat Options Oncol. 2017;18:9.
32. Santini D, Loupakis F, Vincenzi B, et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13:1270-5.
33. Fedyanin M, Stroganova A, Senderovich A, et al. Concordance of KRAS, NRAS, BRAF, PIK3CA mutation status in the primary tumor (PT) and metachronous metastases in patients (pts) with colorectal cancer (CRC). J Clin Oncol. 2016;34(suppl):abstr e15026.
34. Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-6.
35. Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795-801.
36. Taly V, Pekin D, Benhaim L, et al. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin Chem. 2013;59:1722-31.
37. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24.
38. Lee MS, Kopetz S. Current and future approaches to target the epidermal growth factor receptor and its downstream signaling in metastatic colorectal cancer. Clin Colorectal Cancer. 2015;14:203-18.
39. Perkins G, Pilati C, Blons H, Laurent-Puig P. Beyond KRAS status and response to anti-EGFR therapy in metastatic colorectal cancer. Pharmacogenomics. 2014;15:1043-52.
40. Hagemann IS. Molecular testing in breast cancer: a guide to current practices. Arch Pathol Lab Med. 2016;140:815-24.
41. Raghav KPS, Overman MJ, Yu R, et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J Clin Oncol. 2016;34(suppl):abstr 3517.
42. Clark JW, Niedzwiecki D, Hollis D, Mayer R. Phase II trial of 5-fluorouracil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzumab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy. Proc Am Soc Clin Oncol. 2003;22:abstr 3584.
43. Ramanathan RK, Hwang JJ, Zamboni WC, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22:858-65.
44. Leto SM, Sassi F, Catalano I, et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin Cancer Res. 2015;21:5519-31.
45. Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-46.
46. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-8.
47. Liu J, Schmitz JC, Lin X, et al. Thymidylate synthase as a translational regulator of cellular gene expression. Biochim Biophys Acta. 2002;1587:174-82.
48. Jason TL, Berg RW, Vincent MD, Koropatnick J. Antisense targeting of thymidylate synthase (TS) mRNA increases TS gene transcription and TS protein: effects on human tumor cell sensitivity to TS enzyme-inhibiting drugs. Gene Expr. 2007;13:227-39.
49. van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939-50.
50. Chu E, Callender MA, Farrell MP, Schmitz JC. Thymidylate synthase inhibitors as anticancer agents: from bench to bedside. Cancer Chemother Pharmacol. 2003;52(suppl 1):S80-S89.
51. Atkin GK, Daley FM, Bourne S, et al. The impact of surgically induced ischaemia on protein levels in patients undergoing rectal cancer surgery. Br J Cancer. 2006;95:928-33.
52. Qiu LX, Tang QY, Bai JL, et al. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. Int J Cancer. 2008;123:2384-9.
53. Caris Life Sciences. CARIS database. http://www.carislifesciences.com/platforms/caris-research-institute/. Accessed March 18, 2017.
54. Choueiri MB, Shen JP, Gross AM, et al. ERCC1 and TS expression as prognostic and predictive biomarkers in metastatic colon cancer. PLoS One. 2015;10:e0126898.
55. Vincenzi B, Santini D, Tonini G. Thymidylate synthase expression in colorectal cancer: the never-ending story. J Clin Oncol. 2005;23:2108.
56. Butzke B, Oduncu FS, Severin F, et al. The cost-effectiveness of UGT1A1 genotyping before colorectal cancer treatment with irinotecan from the perspective of the German statutory health insurance. Acta Oncol. 2016;55:318-28.
57. Strassburg CP. Pharmacogenetics of Gilbert’s syndrome. Pharmacogenomics. 2008;9:703-15.
58. Etienne-Grimaldi MC, Boyer JC, Thomas F, et al. UGT1A1 genotype and irinotecan therapy: general review and implementation in routine practice. Fundam Clin Pharmacol. 2015;29:219-37.
59. Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA. 1998;95:8170-4.
60. Chen S, Laverdiere I, Tourancheau A, et al. A novel UGT1 marker associated with better tolerance against irinotecan-induced severe neutropenia in metastatic colorectal cancer patients. Pharmacogenomics J. 2015;15:513-20.
61. Yu QQ, Qiu H, Zhang MS, et al. Predictive effects of bilirubin on response of colorectal cancer to irinotecan-based chemotherapy. World J Gastroenterol. 2016;22:4250-8.
62. Shulman K, Cohen I, Barnett-Griness O, et al. Clinical implications of UGT1A1*28 genotype testing in colorectal cancer patients. Cancer. 2011;117:3156-62.
63. Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte-an update of guidelines. Clin Pharmacol Ther. 2011;89:662-73.
64. Dias MM, McKinnon RA, Sorich MJ. Impact of the UGT1A1*28 allele on response to irinotecan: a systematic review and meta-analysis. Pharmacogenomics. 2012;13:889-99.
65. Parker WB, Cheng YC. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther. 1990;48:381-95.
66. Mercier C, Ciccolini J. Profiling dihydropyrimidine dehydrogenase deficiency in patients with cancer undergoing 5-fluorouracil/capecitabine therapy. Clin Colorectal Cancer. 2006;6:288-96.
67. Meulendijks D, Henricks LM, Sonke GS, et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:1639-50.
68. Lee AM, Shi Q, Alberts SR, et al. Association between DPYD c.1129-5923 C>G/hapB3 and severe toxicity to 5-fluorouracil-based chemotherapy in stage III colon cancer patients: NCCTG N0147 (Alliance). Pharmacogenet Genomics. 2016;26:133-7.
69. Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014;32:1031-9.
70. Deenen MJ, Meulendijks D, Cats A, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clin Oncol. 2016;34:227-34.
71. Lunenburg CA, Henricks LM, Guchelaar HJ, et al. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: ready for prime time. Eur J Cancer. 2016;54:40-8.
72. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-6.
73. Des Guetz G, Schischmanoff O, Nicolas P, et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45:1890-6.