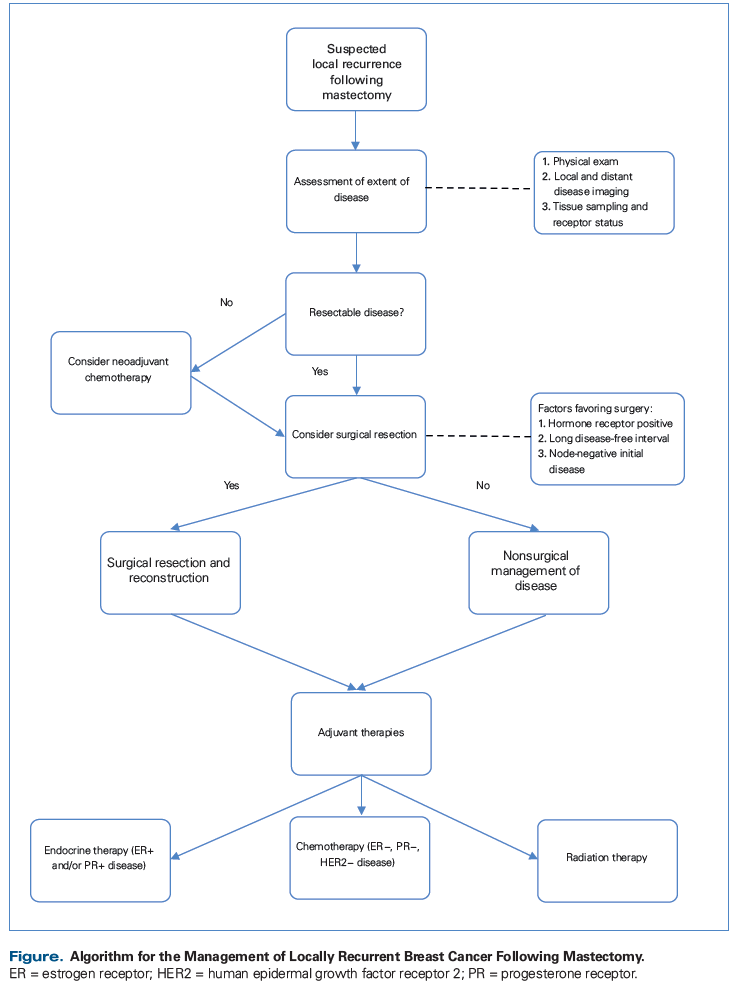
The management of postmastectomy chest wall recurrences of breast cancer has long challenged clinicians. A tissue diagnosis combined with proper imaging and staging of patients to ensure the disease is localized are the first steps in management. Multimodal therapy offers patients the best chances of cure. In properly selected patients, complete surgical resection to negative margins, including full-thickness chest wall resection when required, followed by reconstruction that is well planned, can provide local control with very low surgical mortality and acceptable morbidity. Radiation therapy provides additional local control, while systemic therapy is an adjunct that prolongs survival in many cases. Multidisciplinary care combined with careful patient selection are the keys to successful chest wall resection for locally recurrent breast cancer after mastectomy.
Introduction
Chest wall recurrences of breast cancer not only cause distress to the patient, but also present a challenge to the clinician. These recurrences are often linked to a short disease-free interval combined with high-risk features of the original breast cancer: high nuclear grade, basal phenotype, and advanced stage at presentation. Once considered just a harbinger of distant disease and eventual death from breast cancer, local recurrence of breast cancer after mastectomy is now understood to be treatable, even in cases in which a full-thickness chest wall resection is required. A multidisciplinary approach to these patients, including surgical resection with reconstruction, systemic therapy, and radiation therapy, can often lead to long-term survival.
Initial Workup and Patient Selection
Postmastectomy chest wall recurrences most often manifest as palpable skin-flap masses (96%), either subcutaneous or dermal.[1] The spectrum of clinical severity of locoregional recurrence of breast cancer can extend from a small nodular recurrence along the surgical scar to complete involvement of the chest wall, including the bony thoracic cage, overlying muscles, and soft tissue, and even regional great vessels or nerves. Imaging of the mass with ultrasound and/or MRI helps to define the local extent of the disease process. These imaging studies help in preoperative evaluations and help to identify patients whose disease is unresectable. Following imaging, tissue sampling is paramount to confirm the suspected diagnosis. It is also crucial to ensure proper analysis of the sampled tissue, whether obtained via core biopsy or excisional biopsy. Assessing the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status of a recurrent tumor guides further therapy. In a recent meta-analysis, 20% of ER, 33% of PR, and 8% of HER2 status tumors were discordant with the original tumor.[2] Alterations in the biologic activity of a recurrent breast cancer can markedly change the therapeutic approach to the patient. Careful imaging and definitive tissue diagnosis provide the information required to achieve local control of a recurrent breast cancer.
Prior to initiating therapy, it is important to determine the extent of disease. Buchanan et al, examining their institutional experiences, noted that although the locoregional recurrence after mastectomy was only 8.8%, 36% presented with synchronous metastasis, while an additional 33% would go on to develop distant metastasis.[3] In a recent examination of the National Cancer Database, Neuman et al noted that locoregional recurrence is less common in the era of modern oncologic care, but that its association with distant disease is apparent.[4] Specifically, in patients with a postmastectomy chest wall recurrence, 30% presented with synchronous metastatic disease. In keeping with established guidelines (National Comprehensive Cancer Network [NCCN]), systemic staging is required prior to the consideration of any local therapy. This staging can be in the form of positron emission tomography/computed tomography (PET-CT) or CT chest/abdomen/pelvis combined with a bone scan to exclude metastatic disease. In the absence of systemic disease, the multidisciplinary team can approach the patient with curative intent.
It cannot be emphasized enough that patient selection is the most important factor in determining the utility of full-thickness chest wall resection in the management of a local recurrence of breast cancer (Figure). Patients with surgically resectable disease and no evidence of metastasis can be considered for aggressive local control. Further selection is based on favorable tumor and patient characteristics. Hormone receptor-positive tumors have notably better outcomes following chest wall resection than triple-negative tumors (5-year overall survival [OS] 45% vs 18%).[5] The stage of the original disease can indicate long-term outcomes, with locoregional recurrence of node-negative cancers leading to longer OS.[6] In contrast, patients with tumors that display a more aggressive phenotype through a shortened disease-free interval may not gain a benefit from surgical resection.[6] Patients with poor prognostic indicators-including triple-negative or HER2-positive disease, advanced disease at original diagnosis, and short disease-free intervals-should be approached with caution, because the potential benefit of a full-thickness chest wall resection may be limited (Figure).
Surgical Resection
As noted in the NCCN guidelines, surgical resection is recommended in cases of local recurrence, if possible.[7] Once considered heroic or radical, the full-thickness resection of the chest wall for complete extirpation of a breast cancer recurrence has been notably successful in extending survival in well-selected patients. Several recent single-institution reviews noted complete surgical resection with low mortality, acceptable morbidity, and relatively good quality-of-life outcomes.[8-10] A more recent meta-analysis examined 48 studies, combining morbidity, mortality, survival, and quality-of-life data. The examination of 1,305 patients revealed 30-day morbidity was 20.2% (95% CI, 15.3-26.3) and mortality was less than 1%.[6] Although quality of life was noted to be excellent in 8 studies, the evaluation methods were not standardized. In patients undergoing surgical resection with curative intent, 5-year survival was 41.3% (95% CI, 35.3-47.3). Patients with longer disease-free intervals, hormone receptor-positive tumors, and a lower overall burden of disease had improved survival. Thus, resection of full-thickness chest wall recurrences of breast cancer can clearly provide a survival benefit with acceptable morbidity and mortality.
It has been questioned whether surgery is better than treating the patient with systemic therapy alone. Most studies have pointed to good local control with chest wall resection. There has been little research, however, comparing survival between surgical and nonsurgical groups In a study comparing 44 patients receiving chest wall resection and 32 treated nonsurgically, survival was statistically equivalent.[5] The patients undergoing surgical resection were admittedly well selected, with 95% receiving neoadjuvant therapy either having responsive disease or at least stable disease. The researchers suggest that treating patients with systemic therapy upfront, followed by chest wall resection, may lead to better outcomes.
The strict tenet of surgical resection of isolated chest wall recurrences is negative margins. This is a relatively simple task when the recurrent disease only involves soft tissue (skin, muscle), but it is a more complex task when achieving negative margins requires full-thickness chest wall resection.
Axillary management should be considered largely on a patient-by-patient basis, and the extent to which the axilla is interrogated is determined by clinical history (extent of previous nodal sampling), physical exam, and imaging. Management of the axillary nodes is an extension of local control in this patient population and should be treated as such.
Regardless of the timing of surgery, it is clear that in well-selected patients, surgical resection of their local recurrence can be beneficial. Successful resection of the cancer, however, is only one part of the treatment algorithm. Reconstruction of the resultant defect is often the more complex surgical problem.
Reconstructive Surgery
Planning the reconstruction requires close communication between the oncologic and plastic surgery teams. Preoperative planning involves a discussion of the extent of resection, which can be guided by a review of cross-sectional imaging. Prior radiation treatment can decrease compliance of the chest wall and limit the availability and viability of local tissues and muscles for reconstruction. Previous surgical procedures for locally advanced breast cancer, such as axillary dissection or sternal resection, may damage the vascular pedicle of commonly used muscle flaps, such as the latissimus dorsi or the rectus muscle. To a certain extent, medical comorbidities and overall health will determine the fitness of a patient to undergo longer forms of reconstruction with inherently increased morbidity.
Radical resection of chest wall recurrence may result in a large, full-thickness defect inclusive of soft tissues and underlying skeletal support structures. One of the main concerns in reconstruction of a full-thickness chest wall defect is maintenance of chest wall stability to avoid paradoxical wall motion and disruption of respiratory mechanics. Restoring continuity of the thoracic cage is also important to protect the underlying viscera, such as the heart, lungs, and great vessels. Restoration of soft-tissue coverage of the bony chest is crucial to prevent infection and promote wound healing. The ideal soft-tissue coverage should be compliant, robustly vascularized, and durable. Importantly, the patient should be able to withstand radiation treatment, since many patients with locoregional recurrence will require adjuvant radiotherapy. Finally, the ideal soft-tissue coverage should minimize donor-site morbidity, given that significant complications or problems in wound healing may create an unacceptable delay in the patient's progression to adjuvant therapy.
Intraoperative evaluation of the chest wall defect will determine the final plan for reconstruction. Size of the defect, location on the chest wall, and extent of resection should all be carefully determined. Resection of local chest wall recurrences can involve resection of skin, pectoralis muscle, intercostal muscle, ribs, and sternum. The first important decision is whether restoration of rigid skeletal stability is necessary. In general, the larger the bony defect, the more likely thoracic cage reconstruction will be necessary. Wound diameter greater than 5 cm and resection of more than two ribs are generally considered indications for skeletal fixation. Previous radiation therapy may decrease the risk of paradoxical wall motion due to fibrosis of underlying viscera. Lateral chest wall wounds are more likely to develop instability, since this location lacks support provided by the sternum anteriorly and the spine/scapula posteriorly.[11] Choice of soft-tissue coverage depends on patient factors, defect size, and location, as well as availability of local and regional tissue. Various algorithms can assist in guiding flap choice.[12,13]
Restoration of skeletal stability can be accomplished with placement of mesh or more-rigid constructs. Commonly used materials include synthetic and biologic meshes such as polypropylene, polytetrafluoroethylene, and acellular dermal matrix. When more-rigid fixation is necessary, a common technique involves creation of a methylmethacrylate sandwich between a synthetic mesh bilayer.[14]
Local options for coverage of the chest wall include thoracoepigastric and thoracoabdominal rotation flaps. These fasciocutaneous flaps are based on intercostal or superior epigastric artery perforators and can cover large defects of the chest wall. Local tissues may be damaged and not usable in the setting of previous radiation, and they can create a substantial scar burden on the anterior trunk.[15] Regional options include muscle flaps such as the latissimus dorsi and rectus muscles. Both of these options provide robust, reliable coverage and may be designed as a myocutaneous flap, providing both bulk to fill dead space and replacement for skin. The latissimus flap is based on the thoracodorsal artery pedicle, has a wide arc of rotation, and can be used to resurface the entire ipsilateral chest as well as midline sternal defects. Considered the workhorse of chest wall reconstruction, the latissimus is a versatile option with an acceptably low complication profile.[16] The omentum may even be harvested as a flap based on the gastroepiploic arcade for use in coverage of chest wall defects. Because of the need for intra-abdominal dissection and harvest, omental flaps are usually reserved as a salvage option when regional muscle flaps have failed or are unavailable.
Finally, free tissue transfer can be used in appropriately selected patients. Latissimus muscle, deep inferior epigastric perforator, and anterolateral thigh are potential sources for free tissue transfer. The large number of local and regional options in chest wall coverage, however, means that free tissue transfer is rarely indicated.
Radiation Therapy
Among radiation-naive breast cancer patients who develop a chest wall recurrence, up to 75% will experience this recurrence locally following excision alone, even with widely negative margins.[17-19] Local failure rates can be reduced to approximately 23% to 28% by the addition of adjuvant radiation.[18-20] Radiation also significantly improves distant metastasis-free survival and OS in patients treated for chest wall recurrences.[18,21-23] Complete excision of the recurrence with negative margins coupled with radiation to the entire ipsilateral chest wall and regional lymph nodes is associated with the highest rates of disease-free survival (DFS).[18,22,24,25] Local regional control rates in these patients are not improved by radiation doses higher than 50 Gy, with a 10-Gy boost given in standard fractions.[20,24] For patients with inoperable disease, however, at least 60 Gy is recommended for lesions less than 3.0 cm, and up to 70 Gy is recommended for larger tumors. The addition of concurrent chemotherapy for these patients may or may not improve local control depending on the study, agent used, and tumor subtype. In one study, patients with non-triple-negative tumors had the most benefit from concurrent capecitabine.[22,24,26,27]
Re-irradiation of the chest wall for the treatment of tumor recurrences or new primary tumors has traditionally been avoided for perceived lack of efficacy and fear of toxicity. Several studies have indicated that re-irradiation is not only safe but also may lead to local control rates as high as 60% to 100%, with more favorable outcomes in patients who do not have gross disease at the time of radiation (ie, patients who have had primary surgical treatment with negative margins).[28-30] In these studies, doses between 40 and 60 Gy in standard fractions were administered during the second course of radiation, with the median cumulative radiation dose for both courses being 106 Gy in a multi-institutional study (range, 74.4-137.5 Gy).[28-30] Less than 5% of patients developed grade 3 or 4 acute toxicities, namely moist desquamation, and no patients developed a grade 3 or 4 long-term toxicity.[28-30]
Hyperthermia is a potent radiosensitizer that has been used in combination with radiation for the treatment of chest wall recurrences, including in patients who have been previously irradiated.[31] The most recent studies of hyperthermia have primarily focused on patients with inoperable chest wall disease.[31-36] Among these studies, hyperthermia in combination with radiation improved complete response rates over radiation alone but did not have a significant impact on OS.[27,31-35] This research has been limited by small patient numbers, but a meta-analysis confirmed the complete response rate benefit of hyperthermia, even in patients who have been previously irradiated, with low rates of acute and late grade 3 and 4 toxicities (~14% and ~5%, respectively).[31] Hyperthermia is more effective in tumors that are 5 cm or less in size and when the medial thermal equivalent doses of 42.5°C or 43°C are administered for at least 200 or 100 minutes, respectively.[37,38]
Radiation therapy is an important part of multidisciplinary treatment for operable and inoperable chest wall recurrences of breast cancer and appears to benefit even patients who have been previously irradiated, without significant toxicity.
Systemic Therapy
Considerable debate exists regarding optimal systemic treatment for isolated local or regional recurrence of breast cancer. Although adjuvant and endocrine therapies are well studied regarding their application to primary breast cancers, evidence for these therapies in the setting of disease recurrence is limited. The phase III CALOR (Chemotherapy as Adjuvant for LOcally Recurrent breast cancer) trial, the final analysis of which was recently reported, is perhaps the most well-known trial in this space.[39,40] The trial randomized patients with isolated locoregional recurrence of breast cancer to receive chemotherapy or no chemotherapy, with surgery mandatory and radiation given to those with microscopically involved margins. It demonstrated that adjuvant chemotherapy is beneficial for patients with isolated locoregional recurrence (ILRR) following complete resection among patients with ER-negative malignancy. Five-year DFS in patients who received chemotherapy was greater than that in the nonchemotherapy group (70% vs 34%; hazard ratio [HR], 0.29), when compared with DFS in the ER-positive patients (62% vs 58%; HR, 0.94). A greater trend also was observed toward improved OS with chemotherapy in patients with ER-negative ILRR (73% vs 53% at 10 years) than with chemotherapy in patients with ER-positive ILRR (76% vs 66%). This trial supports the recommendation for adjuvant chemotherapy in patients with ER-negative locoregional recurrences, with much less support for its use in ER-positive patients. The exact chemotherapy regimen is determined by a patient's treatment history, other medical history, and a discussion of risks vs benefits of treatments along with their common side effects and dosing regimens.
KEY POINTS
- Ensure the absence of distant disease before considering surgical resection of a breast cancer chest wall recurrence.
- A multidisciplinary approach to chest wall recurrences is a must.
- An R0 result must be achievable, or surgical resection should not be undertaken.
Although data are limited, endocrine therapy has improved postrecurrence DFS in patients with ER-positive breast cancer. The SAKK 23/82 (Swiss Group for Clinical Cancer Research) study, a long-term analysis of tamoxifen vs observation for patients with ILRR after excision and radiation therapy, demonstrated an increased DFS in the tamoxifen group (61% vs 40%). This increase was most notable in postmenopausal patients (61% vs 33%; P = .006); however, there was no statistically significant improvement in OS. Five-year DFS in premenopausal women was the same in both the tamoxifen and observation groups (60%).[41] Prior analysis of this population (pre- and postmenopausal ER-positive patients) at 6.3 years' median follow-up demonstrated improvement in 5-year DFS from 36% to 59%, which persisted in this long-term study (mean follow-up, 11.6 years) pointing toward overall benefit in regard to DFS.[41,42]
Few trials have evaluated neoadjuvant therapy for ILRR; however, it is often used to improve surgical outcomes by decreasing tumor burden and concomitantly increasing chances of complete resection. Given the association of ILRR both as a marker of metastatic disease and a potential source of new metastases, complete resection is important for optimal and potentially curative treatment.[43,44] Neoadjuvant therapy has the additional advantage of helping to facilitate recognition of early disseminated disease in order to avoid patients having to undergo local treatment unnecessarily.
Systemic therapy, therefore, is part of the standard of care for many patients with local recurrences. Some of these patients will be long-term survivors, but others will develop unresectable metastatic disease despite our best efforts. Regardless, these patients are at higher risk for unwanted side effects from treatment, as well as depression and anxiety related to fear of recurrence. Outcomes for patients with metastatic disease have been shown to improve with early integration of palliative care along with active treatment.[45] We would recommend offering palliative services and psychiatric support to patients with locoregional recurrence, as well as in other situations where these services seem applicable.
Conclusion
A chest wall recurrence of breast cancer following mastectomy is a complex clinical problem that can push centers to the limits of their resources. A comprehensive workup including detailed imaging, staging, and recharacterization of the tumor prepares the patient for possible resection. A complete resection of the recurrence followed by reconstruction of the ensuing defect requires oncologic and plastic surgeons to work together. Adjuvant radiation aids in the control of local recurrence. Systemic therapy also decreases local recurrences and helps to improve OS in these patients. A well-coordinated multidisciplinary approach in well-selected patients with an isolated chest wall recurrence of breast cancer is not only feasible but also desirable for long-term local control and improved survival.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol. 1998;5:620-6.
2. Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50:277-89.
3. Buchanan CL, Dorn PL, Fey J, et al. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg. 2006;203:469-74.
4. Neuman HB, Schumacher JR, Francescatti AB, et al; Alliance/American College of Surgeons Clinical Research Program Cancer Care Delivery Research Breast Cancer Surveillance Working Group. Risk of synchronous distant recurrence at time of locoregional recurrence in patients with stage II and III breast cancer (AFT-01). J Clin Oncol. 2018;36:975-80.
5. Shen MC, Massarweh NN, Lari SA, et al. Clinical course of breast cancer patients with isolated sternal and full-thickness chest wall recurrences treated with and without radical surgery. Ann Surg Oncol. 2013;20:4153-60.
6. Wakeam E, Acuna SA, Keshavjee S. Chest wall resection for recurrent breast cancer in the modern era: a systematic review and meta-analysis. Ann Surg. 2018;267:646-55.
7. National Comprehensive Cancer Network. Breast cancer guidelines, version 1.2018; page 26. https://www.nccn.org/professionals/physicians_gls/pdf/breast.pdf. Accessed July 23, 2018.
8. Friedel G, Kuipers T, Dippon J, et al. Full-thickness resection with myocutaneous flap reconstruction for locally recurrent breast cancer. Ann Thorac Surg. 2008;85:1894-900.
9. Aukema TS, Russell NS, Wesseling J, Rutgers EJ. Extensive soft tissue resection with autologous tissue closure for locally recurrent breast cancer: lasting local control and acceptable morbidity. Eur J Surg Oncol. 2009;35:469-74.
10. van der Pol CC, van Geel AN, Menke-Pluymers MB, et al. Prognostic factors in 77 curative chest wall resections for isolated breast cancer recurrence. Ann Surg Oncol. 2009;16:3414-21.
11. Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804-10.
12. Losken A, Thourani VH, Carlson GW, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg. 2004;57:295-302.
13. Basta MN, Fischer JP, Lotano VE, Kovach SJ. The thoracoplastic approach to chest wall reconstruction: preliminary results of a multidisciplinary approach to minimize morbidity. Plast Reconstr Surg. 2014;134:959e-967e.
14. Lardinois D, Müller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg. 2000;69:919-23.
15. Park JS, Ahn SH, Son BH, Kim EK. Using local flaps in a chest wall reconstruction after mastectomy for locally advanced breast cancer. Arch Plast Surg. 2015;42:288-94.
16. Hameed A, Akhtar S, Naqvi A, Pervaiz Z. Reconstruction of complex chest wall defects by using polypropylene mesh and a pedicled latissimus dorsi flap: a 6-year experience. J Plast Reconstr Aesthet Surg. 2008;61:628-35.
17. Dahlstrom KK, Andersson AP, Andersen M, Krag C. Wide local excision of recurrent breast cancer in the thoracic wall. Cancer. 1993;72:774-7.
18. Bedwinek JM, Fineberg B, Lee J, Ocwieza M. Analysis of failures following local treatment of isolated local-regional recurrence of breast cancer. Int J Radiat Oncol Biol Phys. 1981;7:581-5.
19. Donegan WL, Perez-Mesa CM, Watson FR. A biostatistical study of locally recurrent breast carcinoma. Surg Gynecol Obstet. 1966;122:529-40.
20. Skinner HD, Strom EA, Motwani SB, et al. Radiation dose escalation for loco-regional recurrence of breast cancer after mastectomy. Radiat Oncol. 2013;8:13.
21. Bedwinek J. Radiation therapy of isolated local-regional recurrence of breast cancer: decisions regarding dose, field size, and elective irradiation of uninvolved sites. Int J Radiat Oncol Biol Phys. 1990;19:1093-5.
22. Halverson KJ, Perez CA, Kuske RR, et al. Isolated local-regional recurrence of breast cancer following mastectomy: radiotherapeutic management. Int J Radiat Oncol Biol Phys. 1990;19:851-8.
23. Chagpar A, Kuerer HM, Hunt KK, et al. Outcome of treatment for breast cancer patients with chest wall recurrence according to initial stage: implications for post-mastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 2003;57:128-35.
24. Schwaibold F, Fowble BL, Solin LJ, et al. The results of radiation therapy for isolated local regional recurrence after mastectomy. Int J Radiat Oncol Biol Phys. 1991;21:299-310.
25. Stadler B, Kogelnik HD. Local control and outcome of patients irradiated for isolated chest wall recurrences of breast cancer. Radiother Oncol. 1987;8:105-11.
26. Woodward WA, Fang P, Arriaga L, et al. A phase 2 study of preoperative capecitabine and concomitant radiation in women with advanced breast cancer. Int J Radiat Oncol Biol Phys. 2017;99:777-83.
27. Olson CE, Ansfield FJ, Richards MJ, et al. Review of local soft tissue recurrence of breast cancer irradiated with and without actinomycin-D. Cancer. 1977;39:1981-3.
28. Laramore GE, Griffin TW, Parker RG, Gerdes AJ. The use of electron beams in treating local recurrence of breast cancer in previously irradiated fields. Cancer. 1978;41:991-5.
29. Wahl AO, Rademaker A, Kiel KD, et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys. 2008;70:477-84.
30. Niehoff P, Dietrich J, Ostertag H, et al. High-dose-rate (HDR) or pulsed-dose-rate (PDR) perioperative interstitial intensity-modulated brachytherapy (IMBT) for local recurrences of previously irradiated breast or thoracic wall following breast cancer. Strahlenther Onkol. 2006;182:102-7.
31. Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94:1073-87.
32. Zagar TM, Oleson JR, Vujaskovic Z, et al. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int J Hyperthermia. 2010;26:612-7.
33. Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079-85.
34. Zagar TM, Higgins KA, Miles EF, et al. Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiother Oncol. 2010;97:535-40.
35. Kaidar-Person O, Oldenborg S, Poortmans P. Re-irradiation and hyperthermia in breast cancer. Clin Oncol (R Coll Radiol). 2018;30:73-84.
36. Oldenborg S, Rasch CRN, van Os R, et al. Reirradiation + hyperthermia for recurrent breast cancer en cuirasse. Strahlenther Onkol. 2018;194:206-14.
37. Refaat T, Sachdev S, Sathiaseelan V, et al. Hyperthermia and radiation therapy for locally advanced or recurrent breast cancer. Breast. 2015;24:418-25.
38. Oldenborg S, Griesdoorn V, van Os R, et al. Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: size matters. Radiother Oncol. 2015;117:223-8.
39. Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;15:156-63.
40. Wapnir IL, Price KN, Anderson SJ, et al. Efficacy of chemotherapy for ER-negative and ER-positive isolated locoregional recurrence of breast cancer: final analysis of the CALOR trial. J Clin Oncol. 2018;36:1073-9.
41. Waeber M, Castiglione-Gertsch M, Dietrich D, et al. Adjuvant therapy after excision and radiation of isolated postmastectomy locoregional breast cancer recurrence: definitive results of a phase III randomized trial (SAKK 23/82) comparing tamoxifen with observation. Ann Oncol. 2003;14:1215-21.
42. Borner M, Bacchi M, Goldhirsch A, et al. First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase III multicenter study comparing systemic treatment with observation after excision and radiation. Swiss Group for Clinical Cancer Research. J Clin Oncol. 1994;12:2071-7.
43. Clemons M, Danson S, Hamilton T, Goss P. Locoregionally recurrent breast cancer: incidence, risk factors and survival. Cancer Treat Rev. 2001;27:67-82.
44. Fortin A, Larochelle M, Laverdière J, et al. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J Clin Oncol. 1999;17:101-9.
45. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:96-112.