Neoadjuvant chemotherapy has become the standard of care for patients with locally advanced breast cancer, large tumors, certain biologic subtypes of breast cancer, or locally inoperable disease, and for patients who desire breast conservation. It has the advantage of downstaging the tumor, thereby allowing for conversion from mastectomy to breast conservation, and perhaps decreasing the need for axillary lymph node dissection (ALND). In the past, axillary management involved complete ALND for all patients presenting with breast cancer and involved nodes. With neoadjuvant chemotherapy, some patients exhibit a complete clinical axillary response, which may make them candidates for sentinel lymph node biopsy (SNLB) rather than ALND, with its associated morbidities. While there is widespread use of SLNB in the treatment of breast cancer, its use following neoadjuvant chemotherapy remains widely debated.
Introduction
Surgical management of the axilla in breast cancer has evolved greatly in the last 20 years. Sentinel lymph node biopsy (SLNB), which was first investigated in the early 1990s, has replaced routine axillary lymph node dissection (ALND), with its associated greater morbidity.[1] SLNB provides accurate assessment of nodal status and crucial staging information, it guides additional therapies, and it may replace ALND in a subset of node-positive patients.
Neoadjuvant chemotherapy is increasingly used in the treatment of locally advanced breast cancer, inflammatory breast cancer, large cancers that would otherwise prevent breast-conserving surgery, and some small cancers with aggressive features. In addition, neoadjuvant chemotherapy provides an in vivo evaluation of chemosensitivity to specific agents, as well as assessment of tumor response and potential need for postoperative therapy.[2,3]
Neoadjuvant chemotherapy has survival outcomes similar to those of adjuvant chemotherapy; it may be used for some pathologically node-negative patients and in any patient for whom chemotherapy is planned.[2,4] While there are many obvious advantages to neoadjuvant chemotherapy, a major disadvantage is the confounding of axillary staging, since as many as 40% of patients may convert from pathologically node-positive to pathologically node-negative.[3]
For some patients with limited sentinel node metastases, SLNB, as compared with ALND, has achieved equivalent locoregional recurrence, disease-free survival (DFS), and overall survival (OS) results, without the added morbidity of ALND.[5] However, the role and timing of SLNB, management of the axilla, and technical aspects of the procedure in patients treated with neoadjuvant chemotherapy are matters of controversy. Although data on SLNB and neoadjuvant chemotherapy are accumulating, the value of SLNB in relation to long-term outcomes remains to be seen. This review will discuss the current status of surgical management of the axilla for patients treated with neoadjuvant chemotherapy.
Downstaging After Neoadjuvant Chemotherapy
A number of large randomized studies have shown neoadjuvant chemotherapy to be equivalent to adjuvant chemotherapy with respect to DFS and OS.[3,6-8] In addition, these trials looked at locoregional control after neoadjuvant chemotherapy and indicated that about 10% to 30% of patients who were thought to require mastectomy before neoadjuvant chemotherapy were able to undergo breast-conserving surgery after neoadjuvant chemotherapy.[3,7] In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 trial, one of the early large randomized studies, Fisher et al found that patients who received neoadjuvant chemotherapy had an overall 12% increase in breast-conserving surgery. A majority of patients converting from mastectomy to lumpectomy in this study initially presented with tumors larger than 5 cm.[3] van der Hage et al found a 23% conversion rate from mastectomy to lumpectomy, further demonstrating the utility of neoadjuvant chemotherapy in downstaging primary breast tumors.[7]
Known biologic factors can help determine which patients are most likely to be downstaged after neoadjuvant chemotherapy to allow for breast-conserving surgery. Patients with high-grade breast tumors that are estrogen receptor (ER)-negative and/or human epidermal growth factor receptor 2 (HER2)-positive have a higher likelihood of pathologic complete response (pCR) to neoadjuvant chemotherapy. Rouzier et al found that patients with ER-positive, HER2-negative luminal tumors had only a 6% pCR rate with paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide neoadjuvant chemotherapy, compared with 45% for HER2-positive or basal-like tumors.[9]
The NeoSphere trial investigated the single and combination use of pertuzumab and/or trastuzumab with or without docetaxel in HER2-positive breast cancer. Of the 417 patients randomly assigned to the different arms, 45.8% of patients receiving both pertuzumab and trastuzumab plus docetaxel had a pCR, compared with 29% of those treated with trastuzumab plus docetaxel, 24% of those who received pertuzumab and docetaxel, and 16.8% of those who received only trastuzumab and pertuzumab (P = .0141).[6] Continually increasing pCR is expected with further improvements in systemic therapy.
Nodal response to neoadjuvant chemotherapy
Multiple studies have shown that neoadjuvant chemotherapy can downstage node-positive axillae as well as tumors in the breast, with pCR seen in as many as 40% of patients who were initially node-positive but became node-negative with chemotherapy.[3,4,10] Furthermore, in patients with HER2-positive tumors, the addition of anti-HER2 agents (trastuzumab and pertuzumab) has further improved axillary pCR.[6,11]
Specifically, NSABP B-18 not only examined DFS and OS in patients who received neoadjuvant chemotherapy compared with those treated with adjuvant chemotherapy (the chemotherapy regimen was 4 courses of doxorubicin and cyclophosphamide), but also the effect of neoadjuvant chemotherapy on pathologic and clinical response, axillary downstaging, and conversion from mastectomy to breast-conserving surgery. The study included 747 patients treated with neoadjuvant chemotherapy, of whom 185 had evaluable axillary disease. Of the 185 patients with axillary metastases, 89% had a clinical nodal response to chemotherapy: 73% had a complete clinical response, 16% had a partial clinical response, and 32% had a pCR.[3] Kuerer et al evaluated 191 patients with axillary lymph node metastases who were treated with doxorubicin and subsequently underwent axillary dissection; 23% of the patients demonstrated a nodal pCR.[4] Additionally, Hennessy et al assessed 403 patients with known axillary nodal metastases prior to neoadjuvant chemotherapy and found that 22% of patients achieved pCR. On further analysis, pCR was a predictor of both recurrence-free survival (RFS; 87% in patients with pCR vs 60% in patients with residual disease; P < .0001) and OS (93% in patients with pCR vs 72% in patients with residual disease; P < .0001).[2] If patients with axillary disease at initial presentation can be downstaged, then, ideally, a negative SLNB should prevent the need for ALND.
Identification of Sentinel Lymph Nodes After Neoadjuvant Chemotherapy
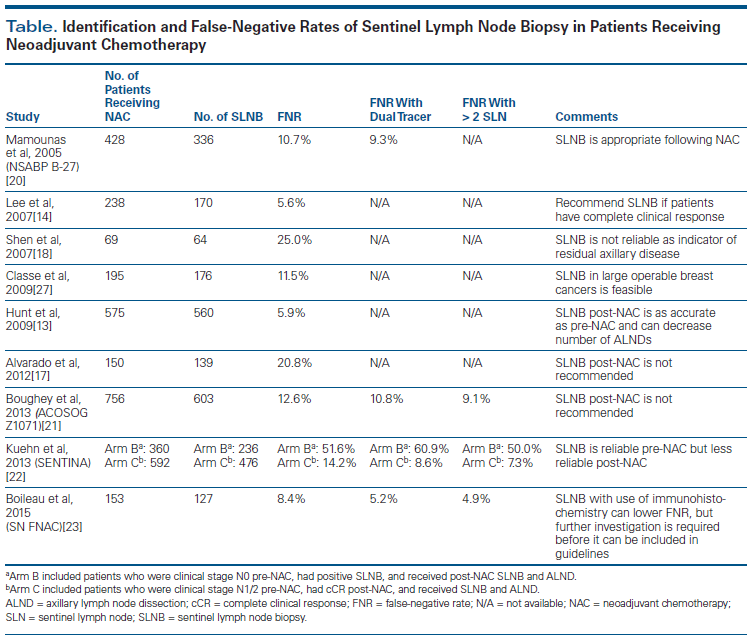
Unfortunately, the reliability of SLNB after neoadjuvant chemotherapy is questionable. While multiple studies have found SLNB to be accurate and adequate for staging after neoadjuvant chemotherapy for patients who were clinically node-negative at presentation, others have noted lower reliability and accuracy (Table).[12-16] In an MD Anderson Cancer Center study, sentinel lymph node identification rates were similar for the 575 women who underwent SLNB after neoadjuvant chemotherapy-97.4%-and for the 3,171 patients treated without neoadjuvant chemotherapy-98.7% (P = .017). False-negative rates (defined as the number of patients with negative SLNB and evidence of residual disease on ALND divided by the total number of patients with residual axillary disease) were also similar: 5.9% vs 4.1% (P = .39).[13] After a median follow-up of 47 months, regional disease recurrence was seen in 0.9% of the patients who underwent SLNB without neoadjuvant chemotherapy, compared with 1.2% in the neoadjuvant chemotherapy group-an insignificant difference. Although no significant differences were seen in sentinel node identification rates or false-negative rates, this study demonstrated that neoadjuvant chemotherapy could be used to downstage disease in the axilla in patients presenting with clinically node-negative T2 and T3 breast tumors, resulting in fewer axillary node dissections without compromising locoregional control.[13] However, patients in this study were extensively screened preoperatively and had axillary ultrasound and often computed tomography or positron emission tomography scans, leading to the selection of patients who for the most part were pathologically node-negative. While the results of this study are helpful for treatment of patients who are node-negative at presentation, it does not address surgical management of the axilla after neoadjuvant chemotherapy in patients who initially present with node-positive disease (either clinical or microscopic). The accuracy of SLNB can only be determined in patients with positive lymph nodes.
Many single-institution studies have shown great variability in the ability to identify the sentinel lymph node, as well as variation in false-negative rates following neoadjuvant chemotherapy, which raises concern about the reliability of the procedure in patients with node-positive breast cancer.[12,14,16-18] Evaluating the accuracy of SLNB in patients with negative axillary nodes is not a true test of its sensitivity, since any node removed at SLNB will be tumor-free and will accurately reflect axillary status. Given this, many patients with suspicious axillary nodes undergo a workup before neoadjuvant chemotherapy, including axillary ultrasound with needle biopsy. For patients identified as having axillary metastases prior to neoadjuvant chemotherapy, National Comprehensive Cancer Network (NCCN) guidelines recommend ALND even if the patient has a response to neoadjuvant chemotherapy.[19]
Application of these guidelines to patients who are clinically node-negative but pathologically node-positive prior to neoadjuvant chemotherapy is supported by earlier studies that show substantial variability in sentinel lymph node identification rates and false-negative rates after neoadjuvant chemotherapy.[12,16-18] Alvarado et al evaluated 150 patients with T1–4, N1–3 breast cancer identified by ultrasound-guided fine-needle aspiration (FNA) who were treated with neoadjuvant chemotherapy and who underwent SLNB followed by ALND. The authors reported a 42% axillary pCR; however, SLNB had a false-negative rate of 20%. Additionally, the false-negative rate increased with removal of fewer nodes, leading the investigators to conclude that SLNB should not be performed in patients presenting with nodal disease even if they have axillary complete clinical response.[17] Shen et al studied 69 patients who had axillary node metastases identified by ultrasound-guided FNA and who underwent SLNB; 61 of these patients underwent concomitant ALND. The investigators found a sentinel lymph node identification rate of 93%; however, the false-negative rate of SLNB was 25% (n = 10).[18] These false-negative rates do not support SLNB in place of ALND in patients with axillary metastases, even with significant response to neoadjuvant chemotherapy. However, these are small studies.
Use of tracer and dye
The NSABP B-27 trial was a large, multicenter study that evaluated SLNB following neoadjuvant chemotherapy. Mamounas et al found a false-negative rate of 11% (15 of 140 patients with negative SLNB), and the sentinel lymph node was successfully identified in 84.8% of the 428 patients undergoing SLNB.[20] The false-negative rate was lowered to 8% when radiocolloid was used in addition to isosulfan blue dye. Furthermore, the investigators found no significant difference in the false-negative rate in patients who presented with node-positive as compared with node-negative disease (7% vs 12.4%, respectively; P = .51), leading them to conclude that patients can safely and accurately undergo SLNB following neoadjuvant chemotherapy regardless of their nodal status at presentation.[20] Other studies show similar results and suggest that results with dual tracer (dye and isotope) are superior to use of either alone.[13,20-23]
Two large prospective studies have investigated SLNB after neoadjuvant chemotherapy. The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial evaluated whether SLNB can accurately identify patients with residual nodal disease following neoadjuvant chemotherapy who had positive axillary nodes at presentation.[21] A total of 701 patients were evaluated, all of whom had clinical N1/2 disease at presentation, confirmed by FNA or core-needle biopsy. Following neoadjuvant chemotherapy, patients underwent SLNB followed by ALND. Identification of at least 1 sentinel node was successful in 92% of patients. The false-negative rate, however, was 12.6% (39 of 310 patients; this only included patients with cN1 disease). The rate was decreased to 10.8% when blue dye plus radiocolloid was used, compared with 20.3% for single-agent use (P = .05). The prespecified endpoint was a false-negative rate less than 10%, and since this was not reached, widespread use of SLNB after neoadjuvant chemotherapy in node-positive patients was not recommended.
Number of sentinel nodes
While the false-negative rate of 10.8% with dual agents is similar to the rates in the original trials of SLNB, current results are significantly better. Among factors identified in ACOSOG Z1071 as contributing to a higher false-negative rate was increased axillary fibrosis after neoadjuvant chemotherapy, which makes lymphatic drainage and surgical dissection more challenging.[21] The authors of this study concluded that changes in approach and patient selection would be necessary to support the use of SLNB as an alternative to ALND.
The Sentinel-Lymph-Node Biopsy in Patients With Breast Cancer Before and After Neoadjuvant Chemotherapy (SENTINA) trial was a prospective multicenter study assessing the optimum timing and accuracy of SLNB in patients undergoing neoadjuvant chemotherapy. Patients who presented with clinical N0 disease (identified via palpation and ultrasound) underwent preoperative SLNB, followed by neoadjuvant chemotherapy (n = 1,022).[22] If the preoperative sentinel lymph node had metastases, then postoperative SLNB with completion ALND was performed (n = 455). Patients with clinical N1/2 disease at presentation received neoadjuvant chemotherapy, and if they were clinically node-negative after neoadjuvant chemotherapy, they underwent SLNB and completion ALND (n = 642). Patients who remained clinically node-positive after neoadjuvant chemotherapy underwent ALND (n = 123). Among clinically node-negative patients, a sentinel lymph node was identified in 99.1% before neoadjuvant chemotherapy, with 35% of those showing nodal metastasis. In these patients in whom nodal metastasis was detected before neoadjuvant chemotherapy, the post–neoadjuvant chemotherapy SLNB only identified the sentinel lymph node in 60.8%, with a false-negative rate of 51% (33 of 64). Patients who were clinically node-positive and who converted to clinically node-negative after neoadjuvant chemotherapy had a sentinel lymph node detection rate of 80.1%, with a false-negative rate of 14.2% (32 of 226 patients). On multivariate analysis of this group, the false-negative rate decreased from 18.5% with 2 sentinel nodes removed to less than 10% when 3 or more sentinel nodes were removed.[22] From this, the authors concluded that selection of patients who can be spared further regional treatment after neoadjuvant chemotherapy remains a clinical challenge.
More recently, a prospective study evaluated the identification rate and false-negative rate of SLNB in patients with biopsy-proven (using FNA or core-needle biopsy) node-positive breast cancer following neoadjuvant chemotherapy.[23] A total of 153 patients with axillary metastases were included; all had clinical examination, ultrasound, and axillary biopsy prior to receiving neoadjuvant chemotherapy. After neoadjuvant chemotherapy, patients underwent SLNB and completion ALND. The identification rate of the sentinel lymph node was 87.6%, with a pCR seen in 34.5% of patients. The false-negative rate was 8.4%, with an average of 2.7 sentinel lymph nodes removed. When only a single sentinel node was removed, the false-negative rate was 18.2%, compared with 4.9% when more than 2 lymph nodes were removed (P = .076). Additionally, use of both isotope and blue dye for identification had a false-negative rate of 5.2%, compared with a false-negative rate of 16.0% (P = .190) for the use of isotope alone. The findings of this study suggest that with adequate lymph node sampling and use of dual tracers, SLNB can be done successfully and accurately in the post–neoadjuvant chemotherapy setting.[23]
The majority of studies evaluating the false-negative rate of SLNB after neoadjuvant chemotherapy looked at identification techniques as secondary outcomes. Virtually all the studies found that the use of radiocolloid or isosulfan blue dye alone was not as successful as the use of both together in identification of the sentinel lymph node.[13,20-23] When attempting SLNB in patients who have previously received neoadjuvant chemotherapy, it may be of value, especially to the inexperienced surgeon, to use dye and isotope to accurately and successfully locate the sentinel lymph node.
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"42393","attributes":{"alt":"","class":"media-image","id":"media_crop_6103874383606","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"4576","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","title":" ","typeof":"foaf:Image"}}]]
William C. Wood, MD
Department of Neurology, Emory University, Atlanta, GeorgiaNew therapies can create new questions.Developments in the Role of Neoadjuvant Chemotherapy …Neoadjuvant chemotherapy for breast cancer has seen a steady rise in use for an increasing number of indications. Widely introduced 40 years ago for locally advanced breast cancer to allow better control by mastectomy and postoperative irradiation, it has progressed to the point that it is considered the ideal timing for anyone who will require chemotherapy. The evidence of response and the reduction in the volume of surgical resection required were gains in themselves. Regression of both the primary tumor and of axillary metastases was appreciated, with many patients rendered yT0 and yN0 after treatment.Together With Developments in Axillary Management...This improvement coincided with-or collided with, depending on one’s point of view-the changing management of the axilla. The senior author of this review and his group introduced breast sentinel lymph node biopsy and have been leaders in the evolving approach to axillary surgery.Create New ChallengesNew questions have arisen regarding the effect of neoadjuvant chemotherapy on axillary evaluation and treatment. These now dominate many tumor board discussions. The issues of timing and effectiveness after neoadjuvant chemotherapy are especially challenging in those with known axillary lymph node metastases prior to neoadjuvant therapy.The authors provide a comprehensive review of the data available to address these questions, offer a reasoned recommendation for the present, and review the ongoing trials that will provide additional information for tomorrow.
Management of the Axilla in Node-Positive Patients After Neoadjuvant Chemotherapy
As would be expected, patients with residual nodal disease following neoadjuvant chemotherapy have a worse prognosis than those with pCR.[2,24] Based on the NCCN guidelines, this subset of patients should undergo ALND and nodal radiation.[19] The real dilemma is not the management of patients with residual nodal disease but the axillary treatment of patients who are node-negative after neoadjuvant chemotherapy. The ACOSOG Z1071 and SENTINA trials did not address clinical outcomes after neoadjuvant chemotherapy and SLNB. If SLNB is not highly accurate, then some patients with a negative sentinel lymph node after neoadjuvant chemotherapy will have residual nodal disease. Since their cancer did not completely respond to chemotherapy, perhaps these are patients for whom axillary dissection would be of value.
ACOSOG Z0011 showed that in patients with T1/2 tumors and limited sentinel lymph node disease (< 3 positive sentinel lymph nodes and no matted nodes or gross extranodal extension), treatment with lumpectomy and radiation was not inferior with respect to OS and DFS compared with treatment with ALND (OS, 92.5% with SLNB alone vs 91.8% with ALND; DFS, 83.9% with SLNB alone vs 82.2% with ALND).[5] The authors concluded that patients with smaller tumors and limited sentinel lymph node disease who will undergo whole-breast irradiation do not benefit from ALND. Unfortunately, this study did not include patients who received neoadjuvant chemotherapy; therefore, its results cannot be applied to patients after neoadjuvant chemotherapy.
The European Organisation for Research and Treatment of Cancer (EORTC) phase III study After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) is a noninferiority trial that randomly assigned over 4,000 patients with T1/2 breast cancer, no palpable lymphadenopathy, and a positive SLNB to either axillary dissection or nodal radiation.[25] Patients were treated with either mastectomy or breast-conserving surgery. Data collection occurred over a 10-year period. The study reported no significant difference in DFS or OS between patients undergoing ALND and those undergoing axillary radiotherapy: DFS was 86.9% vs 82.7%, respectively (P = .18), and 5-year OS was 93.3% vs 92.5%, respectively (P = .34).[25] While this information is helpful, none of the patients in the trial received neoadjuvant chemotherapy, and 71% of patients undergoing ALND had only 1 positive sentinel lymph node, making it difficult to correlate findings to patients with residual disease following neoadjuvant chemotherapy.
Currently, two ongoing sister studies are looking at axillary management following neoadjuvant chemotherapy. Alliance A11202 is a prospective randomized study evaluating the role of ALND in patients with T1–3 N1M0 breast cancer who have sentinel lymph node–positive disease after neoadjuvant chemotherapy. Specifically, radiotherapy (to the breast or chest wall and nodes) plus ALND is being compared with radiotherapy only in this patient population.[21]
NSABP B-51/Radiation Therapy Oncology Group (RTOG) 1304 is evaluating patients with node-positive disease at presentation who have a pCR after neoadjuvant chemotherapy.[26] Patients are randomly assigned to either axillary radiation or no radiation after SLNB shows complete resection of nodal disease. The aim of this trial is to evaluate whether the addition of chest wall and regional nodal radiation after mastectomy or breast-conserving surgery will decrease recurrence in patients converting from node-positive to node-negative disease with neoadjuvant chemotherapy. The results of these studies will assist in clarifying axillary management after neoadjuvant chemotherapy.
Conclusion
While SLNB has replaced ALND for sentinel lymph node–negative patients and some patients with limited axillary metastases, optimal management of the axilla after neoadjuvant chemotherapy is not clear. Avoiding the morbidity of ALND is preferred in any patient who will not derive additional benefit from the procedure; however, it is difficult to accurately identify who these patients are after neoadjuvant chemotherapy. Patients who are clinically node-positive prior to neoadjuvant chemotherapy should undergo ALND, since SLNB may not accurately identify residual axillary disease even if the patient is clinically node-negative after neoadjuvant chemotherapy; currently this is the only way to ensure that appropriate locoregional control is obtained. No study has adequately evaluated outcomes in patients in whom ALND is omitted after neoadjuvant chemotherapy, and further studies are necessary to help determine optimal axillary management.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391-8; discussion 8-401.
2. Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304-11.
3. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on localregional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483-93.
4. Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72-8.
5. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569-75.
6. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25-32.
7. van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224-37.
8. Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96-102.
9. Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678-85.
10. Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93-100.
11. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640-7.
12. Breslin TM, Cohen L, Sahin A, et al. Sentinel lymph node biopsy is accurate after neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2000;18:3480-6.
13. Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558-66.
14. Lee S, Kim EY, Kang SH, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Cancer Res Treat. 2007;102:283-8.
15. Xing Y, Foy M, Cox DD, et al. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539-46.
16. Newman EA, Sabel MS, Nees AV, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007;14:2946-52.
17. Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19:3177-84.
18. Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109:1255-63.
19. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer version 2.2015. J Natl Compr Canc Netw. 2015;13:448-75.
20. Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694-702.
21. Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455-61.
22. Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609-18.
23. Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258-64.
24. Meric F, Mirza NQ, Buzdar AU, et al. Prognostic implications of pathological lymph node status after preoperative chemotherapy for operable T3N0M0 breast cancer. Ann Surg Oncol. 2000;7:435-40.
25. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303-10.
26. Mamounas E. NSABP B-51/RTOG 1304: randomized phase III clinical trial evaluating the role of postmastectomy chest wall and regional nodal XRT (CWRNRT) and post-lumpectomy RNRT in patients (pts) with documented positive axillary (Ax) nodes before neoadjuvant chemotherapy (NC) who convert to pathologically negative Ax nodes after NC. J Clin Oncol. 2014;32(suppl):abstr TPS1141.
27. Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726-32.