Newer Combination Therapies and Targets Reported for Advanced NSCLC
This special “annual highlights” supplement to Oncology News International is acompilation of major advances in the management of lung cancer during 2003, asreported in ONI. Guest editor Dr. Roy Herbst comments on the reports includedherein and discusses advances in the clinical management of lung cancer, with afocus on developments in targeted therapy, new combinations, adjuvant therapy,induction therapy, and what to watch for in 2004.
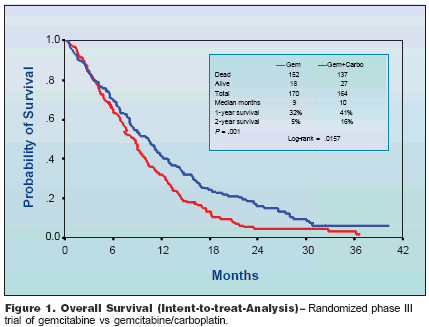
NEW ORLEANS-"Inmetastatic non-small-cell lung cancer,two agents are better than one aslong as one of the agents is a platinumcompound; three drugs are no betterthan two drugs; and carboplatin andcisplatin are similar," according toAlan Sandler, MD, associate professorof medicine at Vanderbilt Universityand director of thoracic oncologyat the Vanderbilt-Ingram CancerCenter.Dr. Sandler spoke at a satellite symposiumheld in conjunction with the44th Annual Meeting of the AmericanSociety for Therapeutic Radiology andOncology (ASTRO). His presentationhighlighted newer combination therapiesas well as chemotherapeutic optionsfor elderly patients and molecularlytargeted agents used in thetreatment of non-small-cell lung cancer(NSCLC).ECOG 1594Dr. Sandler discussed the use ofnewer agents in combination with aplatinum agent for the treatment ofpatients with NSCLC. The results of alarge randomized trial conducted bythe Eastern Cooperative OncologyGroup, ECOG 1594, which comparedgemcitabine (Gemzar)/cisplatin (Platinol),docetaxel (Taxotere)/cisplatin,and paclitaxel/carboplatin (Paraplatin)to the reference arm of paclitaxel/cisplatin, demonstrated no significantdifferences in response rates.Patients who received the gemcitabineand cisplatin regimen, however,demonstrated a significant increasein time to disease progression(4.2 months vs 3.4 months for paclitaxel/cisplatin; P = .001). Dr. Sandlernoted that there was a measurable2-year survival with these regimensthat had not been observed previouslywith combinations of platinums andother agents. Gemcitabine/cisplatindemonstrated the highest 1- and 2-year survival rates (33% and 11%,respectively), but these results werenot statistically significant.Single-Agent vsTwo-Drug CombinationDr. Sandler also addressed whethera single newer third-generation agentwould be just as effective as a twodrugcombination regimen. Data fromthree randomized trials were presentedat the 2002 Annual Meeting of the American Society of Clinical Oncology(ASCO). In these trials, gemcitabine/carboplatin was compared withsingle-agent gemcitabine; paclitaxel/carboplatin was compared with single-agent paclitaxel; and docetaxel/cisplatin was compared with singleagentcisplatin.Gemcitabine/carboplatin was theonly combination that demonstrateda statistically significant difference inoverall survival, compared with single-agent therapy. In this study, gemcitabine/carboplatin produced median,1-year, and 2-year survival of 10months, 41%, and 16%, respectively,compared with 9 months, 32%, and5%, respectively (P = .001), for singleagentgemcitabine (Figure 1)."These three studies," Dr. Sandlerconcluded, "emphasize the fact thatdoublet therapy is in fact better thansingle-agent therapy, and that platinum-based therapy remains the standardof care."Three DrugsNo Better Than TwoGiven the increased efficacy ofsome doublet combination regimensover single-agent therapy, investigatorshave examined whether the additionof a third agent would furtherimprove outcomes. "When you actuallyput it to the test of a randomizedstudy," said Dr. Sandler, "the answer isno, three drugs are not better thantwo-drug regimens."In the first of two randomized trialspresented, gemcitabine/cisplatin wascompared with a three-drug regimenof gemcitabine/cisplatin/vinorelbine(Navelbine). Overall response rate(43.4% vs 38.6%) and median survival(8.7 months vs 7.9 months) wereboth higher with the gemcitabine/cisplatinregimen vs the gemcitabine/cisplatin/vinorelbine regimen.In the second trial, which comparedgemcitabine/carboplatin withMIP (mitomycin [Mutamycin], ifosfamide[Ifex], and cisplatin), the combinationof gemcitabine and carboplatinresulted in a statisticallysignificant increase in median survival,compared with MIP (10 months vs6.5 months, respectively).Carboplatin vsCisplatin SimilarThe relative effectiveness of carboplatinvs cisplatin in the treatment ofpatients with NSCLC has been controversialsince carboplatin was firstintroduced. Two randomized phaseIII trials in patients with stage IIIB orIV NSCLC have recently been completedthat address this issue.In the first trial (TAX 326), patientswere randomly assigned to receivedocetaxel/cisplatin, docetaxel/carboplatin,or vinorelbine/cisplatin. Mediansurvival was 10.9 months in thedocetaxel/cisplatin arm, comparedwith 10.0 months in the control arm ofvinorelbine/cisplatin (P = .122).Median survival in the docetaxel/carboplatin arm was 9.1 months, whichwas not significantly different fromthe control arm. One-year survival was47% in the docetaxel/cisplatin arm,38% in the docetaxel/carboplatin arm,and 42% in the vinorelbine/cisplatinarm. The study was not designed todirectly compare the docetaxel/cisplatinregimen with docetaxel/carboplatin,but to compare each of thesearms with vinorelbine/cisplatin.In a second phase III trial, gemcitabine/cisplatin was compared withgemcitabine/carboplatin. Reported responserates and time to disease progressionwere 47% and 6.1 months,respectively, in the gemcitabine/carboplatinarm compared with 48% and5.6 months in the gemcitabine/cisplatinarm. On the basis of identicalmedian survival (8.1 months in eacharm), Dr. Sandler concluded that thisstudy suggests cisplatin and carboplatinare equivalent when combinedwith gemcitabine in the treatment ofpatients with NSCLC.
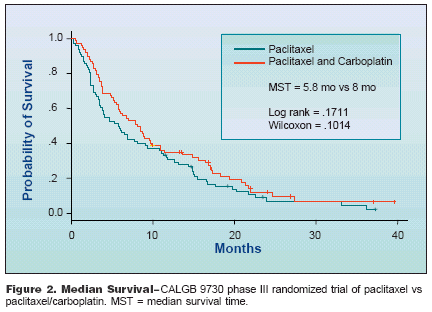
TreatmentConsiderationsDr. Sandler concluded, "Two agentsare better than one, certainly at least aslong as one is a platinum-based agent,and three agents are no better thantwo. The carboplatin/cisplatin debateis on, but I think in metastatic diseaseit is reasonable to consider them similar,although carboplatin certainly hasa better toxicity profile than cisplatin."In randomized trials, the introductionof these newer third-generationchemotherapeutic agents combinedwith a platinum agent has resulted inlonger median, 1-year, and 2-year survivalresults and improved quality oflife compared with older cisplatinbasedregimens. No combination of anewer agent and a platinum agent hasdemonstrated superiority as first-linetherapy in patients with advancedNSCLC; however, toxicity profiles varywith these regimens.Chemotherapyfor ElderlySelecting the most appropriate chemotherapyfor elderly patients withNSCLC is another issue medical oncologistsmust address-namely,whether to treat these patients withsingle-agent therapy or a doublet regimen.A retrospective analysis of astudy conducted by the ECOG, inwhich 15% of the 574 patients enrolledwere ≥ 70 years old, revealed nodifference in response rate or survivalamong this group compared withpatients < 70 years of age.Overall response rates were 21.5%in patients < 70 years old comparedwith 23.3% in patients >= 70. In patients< 70 years old, median survivalwas 9.05 months, and 1- and 2-yearsurvival rates were 38% and 14%, respectively.In patients ≥ 70 years ofage, median survival was 8.53 months,1-year survival was 29%, and 2-yearsurvival was 12%. These results, saidDr. Sandler, suggest that elderly patientswith good performance statusshould be able to tolerate cisplatinbaseddoublet therapy.In a study by the Cancer and LeukemiaGroup B, paclitaxel/carboplatin was compared with single-agentpaclitaxel; 30% of enrolled patientswere > 70 years old and all patientshad a performance status of 0 to 2.Overall survival was 8 months amongpatients who received paclitaxel/carboplatinvs 5.8 months among thosewho received single-agent paclitaxel(Figure 2). Elderly patients with goodperformance status, said Dr. Sandler,should be treated like younger patients,with an appropriate two-drugregimen.Targeting Growth Factors,Signal TransductionMany novel therapies are directedagainst HER1/epidermal growthfactor receptor (HER1/EGFR), which isoverexpressed in 50% to 80% ofNSCLC patients; against vascular endothelialgrowth factor (VEGF), overexpressionof which is associated withdisease progression and decreased survival;or against signal transduction intumor cells.The HER1/EGFR tyrosine kinaseinhibitor erlotinib (OSI-774, Tarceva,investigational) has been studiedin several phase II trials, said Dr.Sandler, including one of 56 patientswith stage IIIb/IV or recurrent metastaticNSCLC who had received at leastone platinum-based therapy. Sevenpatients had a partial response to 150mg/day of erlotinib. Median survivalwas 8.6 months and the 1-year survivalrate was 48%. Acneiform rash wasthe most common adverse event.Phase II trials of ZD1839 (gefitinib,Iressa) IDEAL 1 and IDEAL 2 (IressaDose Evaluation in Advanced LungCancer) found comparable or improvedresponse rates (of approximately20% in IDEAL 1 and 10% inIDEAL 2 patients) and reduced adverseevents with a 250-mg vs 500-mgoral daily dose. In IDEAL 2, diseaserelatedsymptom improvement wasachieved by 43% and 34% of patientsreceiving ZD1839 250 mg/day vs 500mg/day. However, two phase III follow-up trials in chemonaive patientsfound no survival benefit whenZD1839 was added to regimens of paclitaxel/carboplatin or gemcitabine/cisplatin, Dr. Sandler said.In a trial investigating the role ofangiogenesis in NSCLC, the recombinanthumanized monoclonal antibody(rhuMAb) to VEGF was evaluated in99 chemonaive patients with stage IIIb/IV NSCLC who were randomized tocarboplatin/paclitaxel alone or in combinationwith low-dose (7.5 mg/kg) orhigh-dose (15 mg/kg) rhuMAb VEGF.Response rates increased and time totumor progression was prolonged withthe antibody. There were six lifethreateninghemorrhages, apparentlyrelated to squamous cell histology, andan ECOG study is evaluating rhuMAbVEGF in patients without squamouscell tumors.In a recent phase II/III signal transductionblocking study at StanfordUniversity, paclitaxel and carboplatinwere combined with ISIS 3521, an antisenseagent directed against proteinkinase C alpha, which appears to beassociated with the malignant process.The overall response rate was 42%,median survival time was 19 months,and the 1-year survival rate was 75%,Dr. Sandler reported. A randomizedphase III study of 600 patients, comparingcarboplatin/paclitaxel with orwithout ISIS 3521, has recently beencompleted, he said.