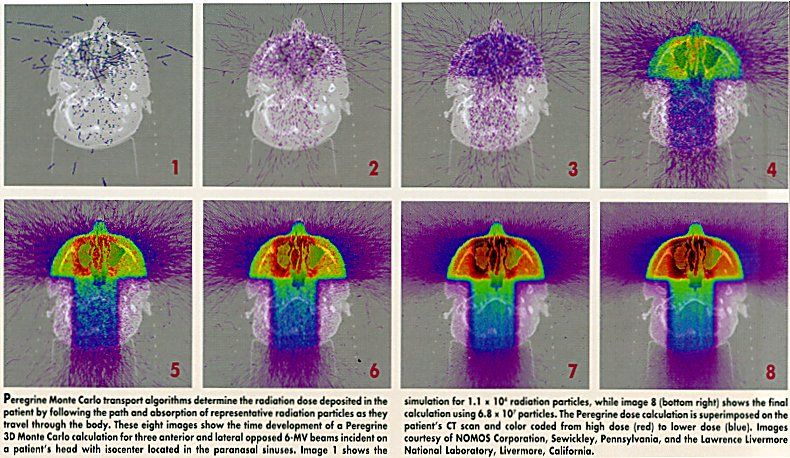
NOMOS Corporation’s Peregrine Radiation Dose Calculation System Approved for Marketing
SEWICKLEY, Pennsylvania-The FDA has granted NOMOS Corporation clearance to market the Peregrine Monte-Carlo-based radiation dose calculation system, licensed from the Lawrence Livermore National Laboratory where it was developed. Peregrine is a computer-based system for quick calculation, in three dimensions, of radiation doses for use with complex intensity-modulated radiotherapy treatment plans. Monte Carlo is a mathematical technique that simulates the trillions of radiation particles that enter the body during treatment. It selects a random sample of these particles and tracks them through a computer model of the radiation-delivery device and the patient’s CT scans to create a detailed map of the dose distribution (see image). NOMOS will initially incorporate Peregrine into its CORVUS inverse treatment planning system, and will then develop a stand-alone version to work with other treatment planning systems.
SEWICKLEY, PennsylvaniaThe FDA has granted NOMOS Corporation clearance to market the Peregrine Monte-Carlo-based radiation dose calculation system, licensed from the Lawrence Livermore National Laboratory where it was developed. Peregrine is a computer-based system for quick calculation, in three dimensions, of radiation doses for use with complex intensity-modulated radiotherapy treatment plans. Monte Carlo is a mathematical technique that simulates the trillions of radiation particles that enter the body during treatment. It selects a random sample of these particles and tracks them through a computer model of the radiation-delivery device and the patients CT scans to create a detailed map of the dose distribution (see image). NOMOS will initially incorporate Peregrine into its CORVUS inverse treatment planning system, and will then develop a stand-alone version to work with other treatment planning systems.