Non-Hodgkin's Lymphoma in the Elderly (Part 1: Overview and Treatment of Follicular Lymphoma)
Non-Hodgkin's lymphoma is one of a few malignancies that have been increasing in incidence over the past several decades. Likewise, these disorders are more common in elderly patients, with a median age of occurrence of 65 years. Therapy in elderly patients may be affected by multiple factors, especially attendant comorbidities. The approaches to management of these patients, with either indolent or aggressive disease processes, have been based on prospective clinical trial results, many of which have included a younger patient population. Fortunately, over the past decade, results of treatment trials that have targeted an older patient population have emerged. The disease incidence and treatment approaches for both follicular (part 1 of this article) and diffuse aggressive (part 2) histologies in elderly patients are reviewed, as well as the impact of aging on the care of these patients.
Non-Hodgkin's lymphoma is one of a few malignancies that have been increasing in incidence over the past several decades. Likewise, these disorders are more common in elderly patients, with a median age of occurrence of 65 years. Therapy in elderly patients may be affected by multiple factors, especially attendant comorbidities. The approaches to management of these patients, with either indolent or aggressive disease processes, have been based on prospective clinical trial results, many of which have included a younger patient population. Fortunately, over the past decade, results of treatment trials that have targeted an older patient population have emerged. The disease incidence and treatment approaches for both follicular (part 1 of this article) and diffuse aggressive (part 2) histologies in elderly patients are reviewed, as well as the impact of aging on the care of these patients.
Non-Hodgkin's lymphoma (NHL) is now the fifth most common malignancy in females, and sixth most common in males.[1] Approximately 59,000 new cases of NHL, and 19,000 deaths due to this disease, are expected in the United States in 2006.[1] Over the past 2 decades, NHL has become one of the few malignancies that is increasing in incidence across all adult age groups, rising by as much as 8% to 10% per year.[2-5] In recent data from the Surveillance, Epidemiology and End Results (SEER) program of the NCI, the incidence of NHL was found to increase exponentially from age 20 to 79 years and then plateau.[6-8] Specifically, the incidence of NHL in US males ranged from 13.1 per 100,000 in people aged 40 to 44 years, to 51.2 per 100,000 in those 60 to 64 years, and 133 per 100,000 in those aged 80 to 84 years.[9]
This increasing incidence is relevant in the elderly population, for although patients aged ≥ 65 years represent 13% of the population, from 25% to 35% of new NHL cases will occur in this group.[10-11] With a median age of 65 years for this disease, about 33% of cases occur in patients who are > 70 years old.[12] With the prediction that the subgroup of the US population > 65 years of age will increase by 12% to 20% over the next several decades, the occurrence of NHL in this older patient population will pose an increasing problem.[13] Incidence rates are higher in males than females across all age groups.[14] In patients older than 60 years of age, incidence rates are slightly higher among whites than blacks. Lastly, the age-specific incidence of small noncleaved cell and lymphoblastic lymphomas increases relatively slowly with age, compared to a more rapid rise in incidence by age for all other histologies.
Part 1 of this two-part article addresses the impact of aging on the treatment of NHL patients, reviews the epidemiology, classification, staging, and prognosis associated with the disease in this setting, and concludes with a discussion of the treatment of follicular NHL. Part 2, which will appear in the September issue of ONCOLOGY, focuses on the treatment of diffuse aggressive lymphomas in the elderly.
Impact of Aging
Clinical care of the older cancer patient is complicated by a variety of factors (Table 1). Clearly, chronologic age alone is not sufficient to categorize these patients.[15] The issue of "ageist stereotyping" may be present among physicians, patients, and family members. Misconceptions with regard to the etiology of cancer, disease course, and treatment may act as barriers to seeking appropriate medical care.[15-17] Specific to elderly NHL patients, a population-based study demonstrated that advanced age was associated with less optimal staging and a greater likelihood of therapy not being administered.[16] Unique social and financial issues related to advanced age also exist in this population.

These issues among others contribute to the paucity of elderly patients in clinical trials. Given a potential referral bias of patients to specialized centers for cancer care, only patients of good performance status (PS) may be entered into many clinical trials. This may not reflect the majority of elderly NHL patients in the general population.[18] There are also issues in evaluating these trials, due to a variable definition of "elderly" (60, 65, or 70 years), small patient numbers, and differing patient characteristics related to PS, comorbidities, and NHL prognostic factors. Quality-of-life assessments assessing both comorbidities and functional status, which are poorly correlated in older cancer patients, are especially important in the elderly population.[17,19-21] Multiple comorbidities have an impact on major organ function and may influence therapy-related toxicities. A correlation has been found between patient function and 2-year mortality.[17] Alterations in host immune function likewise occur in the elderly.[22]
Pharmacokinetics and Pathophysiology
Alterations in pharmacokinetics, including the absorption, distribution, activation, metabolism, and clearance of drugs occur in the elderly patient, related to decreases in total body water, lean body mass, serum albumin, and protein binding, as well as an increase in body fat as one ages.[10,23-24] Hepatic and renal function likewise decline with age, affecting the metabolism and excretion of various chemotherapeutic agents. With advancing age, stem cell reserves may be more rapidly depleted, and tissues may be less capable of repair after therapy-induced damage. In older patients, there is an increased risk of therapy-related myelosuppression, cardiotoxicity, renal insufficiency, neurotoxicity, and mucositis.[25-27] Likewise, issues of "polypharmacy" are often present in these patients, with an increased risk of drug interactions and adverse drug reactions.
Because older NHL patients have a poorer outcome, the question arises as to whether NHL in the older patient is inherently a biologically more aggressive disease than in the younger patient. However, it has been demonstrated in clinical trials that if older patients with diffuse aggressive NHL receive "full dose" chemotherapy, their outcome is comparable to that of younger patients.[28,29] Also in these older patients, death due to nonlymphomatous causes is much more common than in younger patients.[29]
Likewise, the prevalence of NHL histologic subtypes is relatively comparable among younger and older patients, with the exception that Burkitt's and lymphoblastic lymphoma occur more often in younger patients.[30-34] While there is no difference by age associated with disease stage at presentation for the follicular, Burkitt's, or lymphoblastic histologies, advanced-stage disease with diffuse aggressive NHL may be more common in the elderly.[23,30,32,35-37] Although the overall incidence of extranodal NHL does not increase with age, these lymphomas are seen primarily in patients over 60 years old.[30]
Epidemiology, Classification, and Staging in the Elderly
Known risk factors for NHL are not age-specific. These include the presence of functional immune abnormalities, such as autoimmune disorders, congenital immunodeficiencies, and the administration of chronic immunosuppressive therapy. Infections with human immunodeficiency virus, human T-lymphotropic virus types I/II, Epstein-Barr virus, or Helicobacter pylori also increase the risk for developing NHL, as does occupational exposure to agricultural herbicides, pesticides, or industrial solvents.
A series of histologic classifications have been used for NHL. The International Working Formulation (IWF) was developed in 1982, dividing NHL into low-, intermediate-, and high-grade processes, each with characteristic histologies and natural history.[31] However, among other issues with this classification system, it did not account for a variety of recognized histologic subtypes, including mantle cell, monocytoid B-cell, anaplastic large cell, cutaneous T-cell, and primary mediastinal large B-cell NHL. The Revised European American Lymphoma (REAL) classification schema, which incorporated immunologic and molecular genetic aspects along with morphology to better predict clinical course, therapeutic response, and prognosis, was subsequently developed in 1994.[38] This was followed by the World Health Organization (WHO) classification most recently.[39] This system incorporated morphologic, immunophenotypic, and genetic features, as well as clinical aspects, in the classification schema.
The Ann Arbor staging system, which divides NHL into limited-stage (I/II) and advanced-stage (III/IV) disease, has been traditionally used to stage NHL.[40] Staging procedures generally include a complete blood count with differential, lactate dehydrogenase (LDH), computed tomography (CT) scans of the chest, abdomen, and other sites as appropriate, and a bilateral bone marrow biopsy and aspiration. Diagnostic material should be submitted for standard histology, in addition to flow cytometry, immunohistochemistry, and cytogenetic analysis. As there is little morbidity associated with these procedures, they are recommended for patients regardless of age.
Prognostic Factors in the Elderly
The International NHL Prognostic Factors Project (IPI) identified prognostic factors for patients with intermediate/high-grade NHL treated with doxorubicin-based regimens (Table 2).[41] Age-specifically ≥ 60 years-was the most important factor independently associated with outcome, having a negative impact not only on response rate, but also on disease-free and overall survival. Age likewise has been found to be prognostic for survival with these histologies in other series.[42-44]

Other adverse prognostic factors identified by the IPI included an elevated LDH, poor performance status (PS 2-4), advanced-stage disease, and more than one site of extranodal involvement. Four risk subgroups based on these clinical factors were delineated. In addition, for patients with diffuse aggressive NHL, the absence of cell-surface expression of HLA-DR and beta-2–microglobulin, and a higher proliferative rate are associated with a poor response to treatment.[45-49]
For patients with follicular NHL, a similar prognostic schema known as the FLIPI index was devised.[50] The five prognostic parameters identified in this index included age (> 60 vs ? 60 years), Ann Arbor stage (III/IV vs I/II), hemoglobin (< 12 g/dL vs ≥ 12 g/dL), number of involved nodal areas (> 4 vs ? 4), and LDH level (elevated vs normal). The three risk-factor groups subsequently identified were low (0 or 1 adverse factors), intermediate (2 factors), and poor risk (3 or more adverse factors). These parameters had an impact on overall survival in these patients, with 10-year overall survival varying from 71% in the low-risk group, to 51% in the intermediate-risk group, and 36% in the poor-risk group.
In other series of low-grade NHL patients, performance status, Ann Arbor stage, gender, and hemoglobin have been found to be predictive of outcome.[51,52] Advanced age has been prognostic for a poorer outcome in some series[53-56] but not in others.[57-59] Lastly, the prognosis of patients with T-cell processes is generally poorer than that associated with B-cell disorders.
Therapy of Follicular NHL
Approximately 30% to 40% of NHL cases in the elderly are follicular low-grade NHL. Unlike the diffuse aggressive lymphomas, the majority of clinical trials for the therapy of follicular lymphomas include both younger and older patients; therefore, limited data are focused on the elderly. These disorders present insidiously, with B symptoms often absent but generalized lymphadenopathy and hepatosplenomegaly on exam common. Response rates to initial therapy range from 80% to 90%, with complete responses in the majority. Although remissions may last for extended periods, a pattern of continuous late relapse after discontinuation of therapy is present, and cure rates are low. Median survival in the elderly is 5 to 7 years.[60] Prolonged follow-up periods are needed to appropriately assess treatment approaches in these patients.
Patients with limited-stage follicular NHL are generally treated with involved-field radiation therapy, which results in long-term remissions in more than half of patients.[60,61] In one series, there was no difference in the complete response rate by age, and although overall survival was shorter in older patients, the lymphoma-specific death rate did not vary by age.[62] An approach involving no initial therapy has also been examined in this patient population.[63] Although the median age of this group was 58 years, only 17% of patients were 60 years of age or older. With a median follow-up of 86 months, 63% of patients had not yet received therapy. Survival was comparable to historical series of patients receiving immediate treatment.
The majority of follicular lymphoma patients, however, have advanced-stage disease. The option of watchful waiting and therapeutic intervention when symptoms or threatening disease develop may be considered for elderly patients.[64] The approaches to therapy in these patients have evolved over the past decade to include more conventional cytotoxic agents, rituximab (Rituxan)-containing regimens, and the incorporation of radiopharmaceuticals such as tositumumab/iodine-131 tositumomab (Bexxar) or ibritumomab tiuxetan (Zevalin). Other considerations in these patients include quality of life and preservation of active life expectancy as a treatment goal.
Conventional Cytotoxic Therapy
In a large trial conducted by the Cancer and Leukemia Group B (CALGB), single-agent cyclophosphamide was found to result in a similar outcome to anthracycline-based combination chemotherapy in previously untreated follicular lymphoma patients.[65] The median age of the patients enrolled in this study was 56 years; slightly more than one-third of the patients were 60 years of age or older. Not only were the overall response rates and complete response rates comparable among the two treatment arms, but at 10 years of follow-up, the overall time to failure and survival were also comparable. Toxicities were more common in patients receiving the combination therapy. However, in an unplanned subgroup analysis, the patients with follicular mixed NHL who received combination therapy had a significantly better failure-free and overall survival.
Alternatively, fludarabine has been used alone or in combination-as in FND (fludarabine, mitoxantrone, dexamethasone)-in the care of these patients.[66] An additional consideration in these patients is the potential for late cardiac toxicities when anthracyclines are incorporated in the treatment regimen.
Rituximab-Based Therapies
• Single-Agent Rituximab-Single-agent rituximab was initially studied in several phase II trials, first as a salvage therapy option and later as initial therapy for follicular lymphoma patients.[67-72] Significant activity was found in the relapsed/refractory disease setting in these trials, with the median age of patients generally in the mid to late 50s and a significant percentage of patients aged 60 years or older.[67-69] In a subsequent study in which rituximab was employed as first-line and maintanance therapy, the overall response rate was 73%, with 37% complete responses.[70] The median age of patients was 65 years, in a range of up to 89 years.
A European phase III study examined the impact of maintenance rituximab therapy given every 2 months vs observation following initial rituximab treatment.[73] A significant improvement was found in event-free survival among patients who received maintenance therapy vs observation (23 vs 12 months, P = .02), whether previously treated or chemotherapy-naive. The median age of patients enrolled in this trial was 57 years.
The issue of maintanance rituximab therapy vs retreatment at time of disease progression was examined in a phase II trial reported by Hainsworth et al (see Table 3).[74] Not only were final overall and complete responses higher with maintenance therapy, but progression-free survival was also prolonged (31.3 vs 7.4 months, P = .007). However, the duration of benefit with rituximab maintenance therapy and rituximab retreatment was comparable (31.3 vs 27.4 months).
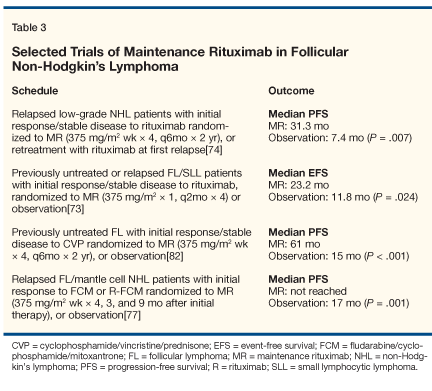
• Rituximab-Chemotherapy Combinations-With the impressive results of single-agent rituximab therapy, additional studies examined the addition of rituximab to established cytotoxic regimens.[75-83] Czuczman et al reported the results of rituximab therapy intially in combination with CHOP (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], prednisone), and later in conjunction with fludarabine, with impressive complete response rates of 63% to 80%.[74,75] A series of phase III trials in which concurrent rituximab plus chemotherapy was compared to chemotherapy alone have been conducted.[77,80-82] In all these trials, it was found that outcome parameters such as response rate, time to treatment failure, and event-free survival were improved by the addition of rituximab to combination chemotherapy.
The approach of four weekly doses of rituximab, followed by short-course chemotherapy with rituximab in combination with either CHOP or CVP (cyclophosphamide, vincristine, prednisone) has also been examined.[83] In this phase II trial, the overall and complete response rates were comparable to rituximab-chemotherapy combinations of longer duration. The Eastern Cooperative Oncology Group (ECOG) chose a different strategy, randomizing patients after induction therapy with CVP to either maintenance rituximab for 2 years or observation, and found that progression-free survival was prolonged by a median of 2.7 years with rituximab maintenance.[82]
In summary, the addition of rituximab to initial chemotherapy-whether concurrently or sequentially as maintenance therapy-or prolonged single-agent rituximab therapy, results in an improved outcome with relation to response and survival. This therapeutic advantage appears to have an impact in both younger and older patients. Although the median age of patients in these series was generally in the 50s, a significant number of patients were at least 60 years of age.
Interferon
Interferon alfa has been utilized in a series of follicular lymphoma studies, not only as part of initial therapy, but also as maintenance therapy, with conflicting results found among these trials. A meta-analysis was undertaken, including 10 phase III trials, in an attempt to clarify the role of interferon in the therapy of these patients.[84] Although the addition of interferon to initial chemotherapy did not improve the overall response rate, it did result in prolonged remission duration. In addition, it prolonged survival in several settings, including when it was given with relatively intensive initial chemotherapy, at a dose of at least 5 million units or at least 36 million units monthly, and when administered with initial chemotherapy as opposed to maintenance therapy.
Radioimmunotherapy
Radioimmunoconjugates have been developed to target the CD20 antigen that is present on most B-cell lymphomas. The two agents that have come into clinical use are 131I-tositumomab and 90Y-ibritumomab.[85,86] The greatest experience with these agents has been in the nonmyeloablative treatment of patients with relapsed or refractory disease, where response rates of 60% to 80% have been achieved. Additional trials have examined the utility of these agents as part of intial therapy, as well as in myeloablative treatment settings.[87,88] The most significant toxicity of these therapies has been myelosuppression, which may be delayed and prolonged. These agents have been utilized in both younger and elderly patients.
Transformation
Transformation of follicular lymphoma to diffuse aggressive NHL, which is heralded by rapid lymph node enlargement, fever, and an elevated LDH, may occur in follicular lymphoma patients. The actuarial incidence of transformation approaches 45% at 18 years after diagnosis.[89] Lymph node biopsy to establish a definitive histologic diagnosis is warranted. Despite the use of more aggressive chemotherapy after transformation, responses generally are of limited duration and outcome is poor.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2006. CA Cancer J Clin 56:106-30, 2006.
2. Devesa SS, Fears T: Non-Hodgkin's lymphoma time trends: United States and international data. Cancer Res 52(suppl 19):5432s-5440s, 1992.
3. Coleman MP, Esteve J, Damiecki P, et al: Trends in cancer incidence and mortality. IARC Sci Publ 121:1-806, 1993.
4. Newton R, Ferlay J, Beral V, et al: The epidemiology of non-Hodgkin's lymphoma: Comparison of nodal and extra-nodal sites. Int J Cancer 72:923-930, 1997.
5. Weisenburger DD: Epidemiology of non-Hodgkin's lymphoma: Recent findings regarding an emerging epidemic. Ann Oncol 5(suppl 1):19-24, 1994.
6. Newell GR, Cabanillas FG, Hagemeister FJ, et al: Incidence of lymphomas in the US classified by the Working Formulation. Cancer 59:857-861, 1987.
7. Rabkin CS, Devesa SS, Zahm SH, et al: Increasing incidence of non-Hodgkin's lymphoma. Semin Hematol 30:286-296, 1993.
8. D'Amore F, Brincker H, Christensen BE, et al: Non-Hodgkin's lymphoma in the elderly. A study of 602 patients aged 70 or older from a Danish population-based registry. Ann Oncol 3:379-386, 1992.
9. National Cancer Institute: SEER Cancer Statistics Review, 1975-2003. Available at http://seer.cancer.gov/cgi-bin/csr/1975_2003/search.pl. Accessed July 12, 2007.
10. Goss PE: Non-Hodgkin's lymphomas in elderly patients. Leuk Lymphoma 10:147-156, 1993.
11. Martelli M, De Sanctis V, Avvisati G, et al: Current guidelines for the management of aggressive non-Hodgkin's lymphoma. Drugs 53:957-972, 1997.
12. Tirelli U, Zagonel V, Errante D, et al: Treatment of non-Hodgkin's lymphoma in the elderly: An update. Hematol Oncol 16:1-13, 1998.
13. Kennedy BJ: Aging and cancer. J Clin Oncol 6:1903-1911, 1988.
14. Groves FD, Linet MS, Travis LB, et al: Cancer surveillance series: Non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst 92:1240-1251, 2000.
15. Berkman B, Rohan B, Sampson S: Myths and biases related to cancer in the elderly. Cancer 74:2004-2008, 1994.
16. Rijke de JM, Schouten LJ, Schouten HC, et al: Age-Specific differences in the diagnostics and treatment of cancer patients aged 50 years and older in the province of Limburg, the Netherlands. Ann Oncol 7:677-685, 1996.
17. McKenna RJ: Clinical aspects of cancer in the elderly. Treatment decision, treatment choices, and follow-up. Cancer 74:2107-2117, 1994.
18. Jelic S, Milanovic N, Tomasevic Z, et al: Comparison of two non-anthracycline-containing regimens for elderly patients with diffuse large-cell non-Hodgkin's lymphoma-possible pitfalls in results reporting and interpretation. Neoplasma 46:394-399, 1999.
19. Yancik R: Cancer burden in the aged. A epidemiologic and demographic overview. Cancer 80:1273-1283, 1997.
20. Extermann M, Overcash J, Lyman GH, et al: Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16:1582-1587, 1998.
21. Lichtman SM: Aggressive lymphoma in the elderly. Crit Rev Oncol Hematol 33:119-128, 2000.
22. Peters FPJ, Ten Haaft MA, Schouten HC: Intermediate and high-grade non-Hodgkin's lymphoma in the elderly. Leuk Lymphoma 33:243-252, 1999.
23. Sweetenham JW, Williams CJ: Malignant lymphoma in the elderly. Baillieres Clin Haematol 1:493-511, 1987.
24. Niitsu N: Non-Hodgkin's lymphoma in the elderly. A guide to drug treatment. Drugs Aging 14:447-457, 1999.
25. Blum RH, Carter SK, Agre K: A clinical review of bleomycin-a new antineoplastic agent. Cancer 31:903-913, 1973.
26. Gobbi PG, Cavalli C: Treatment of adult non-Hodgkin's lymphomas. Haematologica 71:321-344, 1986.
27. Bristow MR, Mason JW, Billingham ME, et al: Doxorubicin cardiomyopathy: Evaluation by phonocardiography, endomyocardial biopsy and cardiac catheterisation. Ann Intern Med 88:168-175, 1978.
28. Dixon DO, Neilan B, Jones SE, et al: Effect of age on therapeutic outcome in advanced diffuse histiocytic lymphoma: The Southwest Oncology Group experience. J Clin Oncol 4:295-305, 1986.
29. Vose JM, Armitage JO, Weisenburger DD, et al: The importance of age in survival of patients treated with chemotherapy for aggressive non-Hodgkin‘s lymphoma. J Clin Oncol 6:1838-1844, 1988.
30. Ballester OF, Moscinski L, Spiers A, et al: Non-Hodgkin's lymphoma in the older person: A review. J Am Geriatr Soc 41:1245-1254, 1993.
31. The Non-Hodgkin's Lymphoma Pathologic Classification Project: National Cancer Institute sponsored study of classification of non-Hodgkin's lymphoma. Summary and description of a working formulation for clinical usage. Cancer 49:2112-2135, 1982.
32. Carbone A, Volpe R, Gloghini A, et al: Non-Hodgkin's lymphoma in the elderly. Cancer 66:1991-1994, 1990.
33. Tirelli U, Zagonel V, Serraino D, et al: Non-Hodgkin's lymphomas in 137 patients aged 70 years or older: A retrospective European Organization for Research and Treatment of Cancer Lymphoma Group study. J Clin Oncol 6:1708-1713, 1988.
34. Neilly IJ, Ogston M, Bennett B, et al: High grade non-Hodgkin's lymphoma in the elderly-12 year experience in the Grampian region of Scotland. Hematol Oncol 13:99-106, 1995.
35. Hoerni B, Sotto JJ, Eghbali H, et al: Non-Hodgkin's malignant lymphomas in patients older than 80. Cancer 61:2057-2059, 1988.
36. Elias L: Differences in age and sex distributions among patients with non-Hodgkin's lymphoma. Cancer 43:2540-2546, 1979.
37. Carbone A, Tirelli U, Volpe R, et al: Non-Hodgkin's lymphoma in the elderly. Cancer 57:2185-2189, 1986.
38. Harris NL, Jaffe ES, Stein H, et al: A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 84:1361-1392, 1994.
39. Harris NL, Jaffe ES, Diebold J, et al: World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 17:3835-3849, 1999.
40. Carbone PP, Kaplan HS, Musshoff K, et al: Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31:1860-1861, 1971.
41. International Non-Hodgkin's Lymphoma Prognostic Factors Project: A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329:987-994, 1993.
42. Hayward RL, Leonard RCF, Prescott RJ: A critical analysis of prognostic factors for survival in intermediate and high grade non-Hodgkin's lymphoma. Br J Cancer 63:945-952, 1991.
43. Velasquez WS, Jagannath S, Tucker SL, et al: Risk classification as the basis for clinical staging of diffuse large-cell lymphoma derived from 10-year survival data. Blood 74:551-557, 1989.
44. Coiffier B, Gisselbrecht C, Vose JM, et al: Prognostic factors in aggressive malignant lymphomas: Description and validation of a prognostic index that could identify patients requiring a more intensive therapy. J Clin Oncol 9:211-219, 1991.
45. Miller TP, Lippman SM, Spier CM, et al: HLA-DR (Ia) immune phenotype predicts outcome for patients with diffuse large cell lymphoma. J Clin Invest 82:370-372, 1988.
46. Swan F, Huh Y, Katz R, et al: Beta-2-microglobulin (?2M) and HLA-DR cellular expression in relapsing large cell lymphomas (LCL): Relationship to survival and serum ?2M levels (abstract). Blood 76(suppl 1):375a, 1990.
47. Bauer KD, Merkel DE, Winter JN, et al: Prognostic implications of ploidy and proliferative activity in diffuse large cell lymphomas. Cancer Res 46:3173-3178, 1986.
48. Grogan TM, Lippman SM, Spier DM, et al: Independent prognostic significance of nuclear proliferation antigen in diffuse large cell lymphomas as determined by the monoclonal antibody Ki-67. Blood 71:1157-1160, 1988.
49. Wooldridge TN, Grierson HL, Weisenburger DD, et al: Association of DNA content and proliferative activity with clinical outcome in patients with diffuse mixed cell and large cell non-Hodgkin's lymphoma. Cancer Res 48:6608-6613, 1988.
50. Solal-Celigny P, Roy P, Colombat P, et al: Follicular lymphoma international prognostic index. Blood 104:1258-1265, 2004.
51. Leonard RCF, Hayward RL, Prescott RJ, et al: The identification of discrete prognostic groups in low grade non-Hodgkin's lymphoma. Ann Oncol 2:655-667, 1991.
52. Soubeyran P, Eghbali H, Bonichon F, et al: Low-grade follicular lymphomas: Analysis of prognosis in a series of 281 patients. Eur J Cancer Clin Oncol 27:1606-1613, 1991.
53. Romaguera JE, McLaughlin P, North L, et al: Multivariate analysis of prognostic factors in stage IV follicular low grade lymphoma: a risk model. J Clin Oncol 9:762-769, 1991.
54. Weisdorf DJ, Andersen JW, Glick JH, et al: Survival after relapse of low-grade non-Hodgkin's lymphoma. Implications for marrow transplantation. J Clin Oncol 10:942-947, 1992.
55. Paryani SB, Hoppe RT, Co RS, et al: Analysis of non-Hodgkin's lymphomas with nodular and unfavourable histologies, stages I and II. Cancer 52:2300-2307, 1983.
56. Rudders RA, Kaddis M, Delellis RA, et al: Nodular non-Hodgkin's lymphoma (NHL): Factors influencing prognosis and indicators for aggressive treatment. Cancer 43:1643-1651, 1979.
57. Gallagher CJ, Gregory WM, Jones AE, et al: Follicular lymphoma: Prognostic factors for response and survival. J Clin Oncol 4:1470-1480, 1986.
58. Cabanillas F, Smith T, Bodey GP, et al: Nodular malignant lymphoma: factors affecting complete response rate and survival. Cancer 44:1983-1989, 1979.
59. McLaughlin P, Fuller LM, Velasquez WS, et al: Stage I-II follicular lymphoma. Treatment results for 76 patients. Cancer 58:1596-1602, 1986.
60. O'Reilly S, Connors JM, Macpherson N, et al: Malignant lymphomas in the elderly. Clin Geriatr Med 13:251-263, 1997.
61. MacManus MP, Hoppe R: Is radiotherapy curative for stage I and stage II low grade follicular lymphoma? Results of a long term follow-up study of patients treated at Stanford University. J Clin Oncol 14:1282-1290, 1996.
62. Tezcan H, Vose JM, Bast M, et al: Limited stage I and II follicular non-Hodgkin's lymphoma: The Nebraska Lymphoma Study Group experience. Leuk Lymphoma 34:273-285, 1999.
63. Advani R, Rosenberg SA, Horning SJ: Stage I and II follicular non-Hodgkin's lymphoma: Long-term follow-up of no initial therapy. J Clin Oncol 22:1454-1459, 2004.
64. Connors JM, O'Reilly SE: Treatment considerations in the elderly patient with lymphoma. Hematol Oncol Clin North Am 11: 949-960, 1997.
65. Peterson BA, Petroni G, Frizzera G, et al: Prolonged single-agent versus combination chemotherapy in indolent follicular lymphomas: A study of the Cancer and Leukemia Group B. J Clin Oncol 22:5-15, 2003.
66. McLaughlin P, Hagemeister F, Romaguera J, et al: Fludarabine, mitoxantrone, and dexamethasone: An effective new regimen for indolent lymphoma. J Clin Oncol 14:1262-1272, 1996.
67. Maloney DG, Grillo-Lopez AJ, White CA, et al: IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90:2188-2195, 1997.
68. McLaughlin P, Grillo-Lopez AJ, Link BK, et al: Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825-2833, 1998.
69. Feuring-Buske M, Kneba M, Unterhalt M, et al: IDEC-C2B8 (rituximab) anti-CD20 antibody therapy in relapsed advanced-stage follicular lymphomas: Results of a phase-II study of the German Low-Grade Lymphoma Study Group. Ann Hematol 79:493-500, 2000.
70. Hainsworth JD, Litchy S, Burris HA, et al: Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin's lymphoma. J Clin Oncol 20:4261-4267, 2002.
71. Columbat P, Salles G, Brousse N, et al: Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood 97:101-106, 2001.
72. Witzig TE, Vukov AM, Habermann TM, et al: Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade 1 non-Hodgkin's lymphoma: A phase II trial in the North Central Cancer Treatment Group. J Clin Oncol 23:1103-1108, 2005.
73. Ghielmini M, Schmitz SFH, Cogliatti SB, et al: Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response durauion compared with the standard weekly x 4 schedule. Blood 103:4416-4423, 2004.
74. Hainsworth JD, Litchy S, Shaffer DW, et al: Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma-a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 23:1088-1095, 2005.
75. Czuczman MS, Weaver R, Alkuzweny B, et al: Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9 year follow-up. J Clin Oncol 22:4711-4716, 2004.
76. Czuczman MS, Koryzna A, Mohr A, et al: Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol 23:694-704, 2005.
77. Forstpointer R, Dreyling M, Repp R, et al: The additon of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared to FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of the German Low Grade Lymphoma Study Group (GLSG). Blood 104:3064-3071, 2004.
78. Hiddemann W, Kneba M, Dreyling M, et al: Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 106:3725-3732, 2005.
79. Marcus R, Imrie K, Belch A, et al: CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 105:1417-1423, 2005.
80. Herold M, Pasold R, Srock S, et al: Results of a prospective randomized open label phase III study comparing rituximab plus mitoxantrone, chlorambucil, prednisolone chemotherapy (R-MCP) to MCP alone in untreated advanced indolent non-Hodgkin's lymphoma (NHL) and mantle cell lymphoma (MCL) (abstract 584). Blood 104:169a, 2004.
81. Salles G, Foussard C, Nicolas M, et al: Rituximab added to aIFN + CHVP improves the outcome of follicular lymphoma patients with a high tumor burden: First analysis of the GELA-GOELAMS FL-2000 randomized trial in 359 patients (abstract 160). Blood 104:49a, 2004.
82. Hochster HS, Weller E, Gascoyne RD, et al: Maintenance rituximab after CVP results in superior clinical outcome in advanced follicular lymphoma (FL): Results of the E1496 phase III trial from the Eastern Cooperative Oncology Group and the Cancer and Leukemia Group B. Blood 106:349a, 2005.
83. Hainsworth JD, Litchy S, Morrissey LH, et al: Rituximab plus short-duration chemotherapy as first-line treatment for follicular non-Hodgkin's lymphoma: A phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 23:1500-1506, 2005.
84. Rohatiner AZS, Gregory WM, Peterson B, et al: Meta-analysis to evaluate the role of interferon in follicular lymphoma. J Clin Oncol 23:2215-2223, 2005.
85. Kaminski MS, Estes J, Zasadny KR, et al: Radioimmunotherapy with iodine (131) tositumomab for relapsed or refractory B-cell non-Hodgkin's lymphoma: Updated results and long-term follow-up of the University of Michigan experience. Blood 96:1259-1266, 2000.
86. Witzig TE, Gordon LI, Cabanillas F, et al: Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol 20:2453-2463, 2002.
87. Kaminski MS, Tuck M, Estes J, et al: 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med 352:441-449, 2005.
88. Press OW, Unger JM, Braziel RM, et al: Phase II trial of CHOP chemotherapy followed by tositumomab/iodine-131 tositumomab for previously untreated follicular non-Hodgkin's lymphoma: Five-year follow-up of Southwest Oncology Group protocol S9911. J Clin Oncol 24:4143-4149, 2006.
89. Horning SJ: Low-grade lymphoma 1993: State of the art. Ann Oncol 5(suppl 2):S23-S27, 1994.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.