Novel Drug, MDV3100, Will Likely Have a Major Role in Prostate Cancer Treatment
The new drug, MDV3100, extended overall survival by 4.8 months (P < .001) and reduced the risk for death by 37% as compared to placebo in men with castration-resistant prostate cancer who had progressed after treatment with docetaxel.
The new drug, MDV3100, extended overall survival by 4.8 months (P < .001) in men with castration-resistant prostate cancer who had progressed after treatment with docetaxel. It also reduced the risk for death by 37% as compared to placebo. This is an impressive feat. Currently, there is no standard of care for this group of patients.
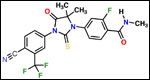
Chemical structure of the antiandrogen MDV3100
MDV3100 is an oral, androgen receptor signaling inhibitor that competitively inhibits the binding of androgens to the androgen receptor, and uniquely inhibits the receptor from translocating to the nucleus and binding DNA. MDV3100 was chosen for development based on robust prostate cancer model systems and activity in early stage trials in both chemotherapy-treated and chemotherapy-naive prostate cancer patients. It is the first in a new class of agents and different from the mechanism of action of currently available treatments.
The data, part of the AFFIRM study, was presented by Dr. Howard I. Scher, chief of the genitourinary oncology service at Memorial Sloan-Kettering Cancer Center, a late-breaking presentation at the 2012 American Society of Clinical Oncology Genitourinary Cancers Symposium.
In total, 1199 patients were randomized over a 1-year time period starting in 2009. Patients had advanced disease, about 90% with bone metastases and 70% with soft tissue metastases.
The estimated median overall survival was 18.4 months for the MDV3100-treated patients compared to 13.6 months for patients treated with placebo. MDV3100 treatment was also associated with a 50% reduction in prostate-specific antigen (PSA) levels in 54% of MDV3100-treated patients compared with 1.5% of placebo patients. The median time to PSA progression was 8.3 months in the experimental group compared to 3 months in the untreated group.
Safety was also favorable, with mild adverse events, mostly fatigue, diarrhea, and hot flashes, which were reported in about 2% of patients in both the experimental and placebo arms. One potential concern is a rare incidence of seizure seen in 5 patients in the MDV3100 arm. According to the trial authors, these cases were studied carefully and could be due to confounding events including brain metastases.
The drug is made by Medivation in collaboration with Astellas. There is an ongoing clinical trial testing the efficacy of MDV3100 in prostate cancer patients who have not yet received docetaxel.
Abiraterone (Zytiga) and cabazitaxel (Jevtana) have also recently showed a benefit in survival in phase III trials among prostate cancer patients after a docetaxel regime. Cabazitaxel, a microtubule inhibitor, was approved by the Food and Drug Administration in 2010. The trial that led to the approval showed a 30% reduced risk of death and improved overall survival by 2.4 months for cabazitaxel combined with prednisone. Abiraterone, a steroidal compound with antiandrogen activity, was approved this April, in combination with prednisone, demonstrating a survival benefit of 3.9 months.