As care for the childhood cancer patient has improved significantly, there is an increasing incidence of treatment-related late effects. Obesity and type 2 diabetes mellitus are common and significant metabolic conditions in some populations of adult survivors of childhood cancer. Results from the Childhood Cancer Survivor Study and other large cohorts of childhood cancer survivors reveal that long-term survivors of acute lymphoblastic leukemia and those who received total body irradiation or abdominal radiotherapy are at highest risk. The potential mechanisms for the observed increase in risk, including alterations in leptin and adiponectin, pancreatic insufficiency, poor dietary habits, sedentary lifestyle, and perhaps changes in the composition of the gut microbiota, are reviewed. Discussion of exercise and diet intervention studies shows that further research about the barriers to a healthy lifestyle and other interventions in childhood cancer survivors is warranted.
Introduction
Over the past few decades, diagnosis, treatment, and supportive care for childhood cancer have improved significantly.[1] For contemporary childhood cancer patients in the United States, the possibility of cure now exceeds 80%.[2] These successes have resulted in an estimated 420,000 survivors of cancer diagnosed prior to age 21 who are living in the United States.[3] In many ways, acute lymphoblastic leukemia (ALL), the most common childhood cancer, represents the prototypical childhood cancer. ALL was uniformly fatal as little as a generation ago,[4,5] yet current 5-year survival for children with ALL is 90% or greater.[1,2] Unfortunately, long-term survivors of childhood cancer, including ALL survivors, are at lifelong risk for treatment-related late effects.[6-8] Indeed, while children with ALL have an excellent chance of cure, the conditional life expectancy for a 5-year survivor of childhood ALL is only 54.7 years.[9]
Obesity, hyperlipidemia, and diabetes mellitus (DM) are among the most common and significant late effects among childhood cancer survivors. Results from the Childhood Cancer Survivor Study (CCSS) and other large cohorts of childhood cancer survivors reveal that long-term survivors of ALL and those who received total body irradiation (TBI) or abdominal radiotherapy (RT) are at highest risk for these late effects.[10] In this review, childhood cancer survivor populations at risk for metabolic late effects, as well as mechanisms and ongoing intervention studies, will be detailed.
At-Risk Populations
ALL survivors
ALL is the most common childhood cancer, with approximately 3,000 new cases diagnosed each year[11,12] and an estimated 50,000 adult survivors of childhood ALL currently living in the United States.[13,14] As previously noted, in spite of these excellent disease-specific survival rates, adult survivors of childhood ALL are at significant risk for late-occurring cardiovascular and metabolic diseases, including obesity, DM, hypertension, and hyperlipidemia.[15,16] In a cross-sectional comparison of 118 adult survivors of childhood ALL and regional controls, female ALL survivors with a history of cranial radiotherapy (CRT) had higher levels of fasting insulin and glucose, were more insulin-resistant as measured by the Homeostasis Model for Assessment of Insulin Resistance (HOMA-IR), and were more likely to have two or more cardiovascular risk factors than the regional controls.[17] Analysis via dual-energy x-ray absorptiometry (DXA) revealed that ALL survivors with a history of CRT also had lower lean body mass and increased abdominal and visceral fat mass compared with community controls.[18]
Dyslipidemia appears to be another important component of metabolic disease among adult survivors of childhood ALL. An analysis of lipid subfractionation among 110 long-term survivors of childhood ALL was recently published. Although mean low-density lipoprotein cholesterol (LDL-c) levels were normal (108.7 mg/dL), 36% of survivors had more than 50% of LDL-c in small, dense, more atherogenic LDL subfractions.[19] Furthermore, survivors with the atherogenic LDL pattern had lower high-density lipoprotein (HDL), higher triglycerides, higher body mass index (BMI), more visceral adiposity, and more insulin resistance, and were more likely to have metabolic syndrome vs survivors without the atherogenic lipid pattern.
As previously noted, CRT is an important risk factor for cardiometabolic disease among ALL survivors. In the CCSS, ALL survivors treated with ≥ 20 Gy CRT had an increased chance of being obese (odds ratio [OR], 2.59 for females [95% confidence interval (CI), 1.88–3.55]; P < .001; and OR, 1.86 for males [95% CI, 1.33–2.57]; P < .001) compared with sibling controls. Female survivors treated before the age of 5 and with ≥ 20 Gy CRT were at highest risk (OR, 3.81 [95% CI, 2.34–5.99]; P < .001) compared with sibling controls.[20] CRT also increases the risk of a diagnosis of DM; the OR for a diagnosis of DM among male and female ALL survivors in the CCSS with a history of CRT compared with sibling controls was 1.8 (95% CI, 1.2–2.8; P < .01). After adjusting for BMI, the OR for diagnosis of DM was 1.6 (95% CI, 1.0–2.5; P < .06) among survivors with a history of CRT compared with sibling controls.[21] Importantly, the contemporary use of CRT is limited, yet the prevalence of childhood obesity has increased dramatically over the past 35 years. Today, many children are obese at the time of diagnosis, and so are likely to remain at risk for becoming obese long-term survivors despite non-irradiating therapies. Recent work by Zhang et al[22] suggests that the excessive weight gain occurs early in therapy, regardless of age, sex, or degree of obesity at the time of diagnosis.
Total body irradiation
TBI has been used for many years as part of the conditioning regimen for stem cell transplants. The radiation is usually delivered at a fractionated dose of 1,200 to 1,500 cGy. In 1995, Lorini et al[23] described a state of insulin resistance in survivors who were 1-year post–bone marrow transplant. Insulin levels after an intravenous glucose tolerance test were significantly higher in the patients that had been conditioned with TBI.
Since then, larger cohorts have corroborated this finding.[16,21,24,25] In the CCSS, using self-reported outcomes of 8,599 childhood cancer survivors, TBI was an independent risk factor for the development of DM and dyslipidemia compared with siblings, with an adjusted OR of 7.8 (95% CI, 3.5–16.4) and 3.9 (95% CI, 1.8–7.6), respectively.[16] Oudin and colleagues assessed the prevalence of metabolic syndrome in a French multicentric program of leukemia survivors.[26] Interestingly, they found TBI to be a major risk factor for the development of dyslipidemia (hypertriglyceridemia and low HDL) and hyperglycemia, without a significant influence on waist circumference or hypertension. These findings are consistent with other studies describing non-obese survivors exhibiting metabolic syndrome or some of its components.[27]
One long-term follow-up of pediatric stem cell transplant survivors demonstrated that the risk of hypertension in TBI-exposed survivors was twice that of survivors not exposed to TBI.[28] The 30-year cumulative incidence of hypertension was 40% in the TBI group. These metabolic aberrations put TBI-exposed survivors at risk for premature cardiovascular aging. In the CCSS cohort, survivors who reported having three or four cardiovascular risks were at increased risk for cardiac events.[16] One hypothesis explaining this paradox is the possible deleterious effect of TBI on body composition, resulting in sarcopenic obesity-the loss of muscle mass in combination with an increase in visceral adipose tissue.[29] This, in turn, could induce insulin resistance without an increase in BMI.
Abdominal radiation
Abdominal radiation is an integral tool in the treatment of many childhood cancers, including neuroblastoma, Wilms tumor, soft tissue sarcomas, and germ cell tumors. Historically, patients with Hodgkin lymphoma were also treated with infradiaphragmatic radiation to the spleen or para-aortic fields, although this approach is used infrequently in contemporary protocols.
A number of recent reports have highlighted the risk of DM after abdominal RT in adult survivors of childhood cancer. This association was first noted in 1995 when Teinturier et al reported a 6.6% prevalence of non-autoimmune “pancreatic DM” in 121 young adult Wilms tumor survivors treated with abdominal RT during childhood.[30] Associations between abdominal RT and impaired pancreatic β-cell function were subsequently noted in other small cohorts of childhood cancer survivors in the United Kingdom and Italy.[31,32]
In 2009, the first large study on DM risk in 8,599 CCSS childhood cancer survivors and their 2,936 siblings found that survivors were 1.8 times more likely than their siblings to report DM, after adjusting for BMI, age, sex, race/ethnicity, household income, and insurance status.[21] In multivariate models, exposure to abdominal RT and TBI was a significant predictor of risk. Similarly, in a population-based study of 32,903 1-year survivors of childhood cancer from the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) cohort, the authors noted a 1.6-fold increased risk of DM-related hospital contact for survivors of childhood cancer when compared with the general population; risk increased with increasing time from completion of RT.[33]
Two large European cohort studies have since used radiation dosimetry to explore a possible association between abdominal radiation dose and subsequent risk of DM. In a retrospective cohort of 2,520 childhood cancer survivors treated in France and the United Kingdom, de Vathaire et al noted a dose-response relationship between radiation exposure to the tail of the pancreas, where insulin-producing cells reside, and subsequent risk of DM through 20 to 29 Gy.[34] More recently, an analysis of 2,264 Hodgkin lymphoma survivors in the Netherlands found an increased risk of DM among survivors; those treated with ≥ 36 Gy to the para-aortic lymph nodes and spleen (which includes 90% to 100% of pancreatic volume) were at highest risk.[35] Risk increased significantly with higher mean radiation doses to the pancreatic tail.
Mechanisms
In describing mechanisms, current literature will be reviewed here; however, not much is known about the pathophysiology of obesity and metabolic syndrome in the childhood cancer survivor population.
Leptin resistance and hypothalamic dysfunction
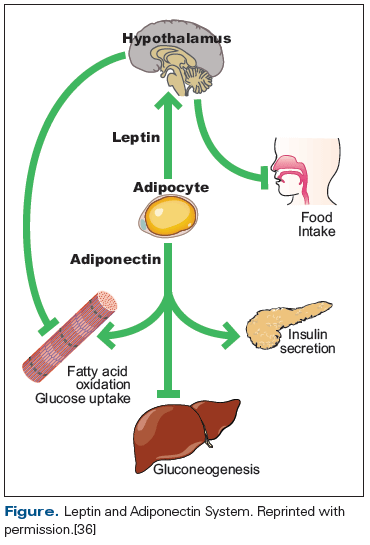
Alterations in leptin and adiponectin may contribute to obesity and metabolic disease among childhood cancer survivors. Leptin and adiponectin are peptide hormones secreted by adipocytes (Figure).[36] Leptin is proportional to total body fat mass and communicates primarily with the hypothalamus regarding satiety and energy usage. Adiponectin is inversely proportional to fat mass and appears to communicate primarily with skeletal muscle and the liver, with respect to energy storage. In a cross-sectional analysis of 116 ALL survivors, higher leptin and lower adiponectin were associated with insulin resistance and other measures of body fat, suggesting central leptin resistance.[36] A biologic interaction between leptin receptor activity, CRT, and obesity may exist; a polymorphism of leptin receptor (LEPR GlnQ223Arg) interacts with CRT in its association with insulin resistance and is independently associated with obesity among female ALL survivors.[37]
Pancreatic insufficiency
The pancreas maintains both endocrine and exocrine functionality. As an endocrine organ, the islet cells of the pancreas release insulin and glucagon into the bloodstream and thereby maintain glucose homeostasis by promoting glucose uptake by the skeletal muscle, liver, and adipose tissue. Preclinical studies on the rat and primate pancreas have demonstrated marked pancreatic histologic changes after RT, including necrosis of the islet cells, degranulation, vacuolization, mitochondrial destruction, and an increase in lysosomes.[38,39] Significant pathologic changes to the canine pancreas have also been noted after RT.[40,41]
In addition to preclinical data, multiple clinical studies have postulated a link between radiation-induced damage to the β cells of the pancreas and risk of DM.[31,34,35] Yet it is unlikely that pancreatic insufficiency alone mediates this risk; Oeffinger and Sklar have theorized that increased visceral adiposity after RT, which is known to be associated with insulin resistance, also contributes to DM risk.[42] Muscle may also play an important part in the pathogenesis of DM in childhood cancer survivors. A recent study on type 2 DM risk in nonhuman primates after whole-body irradiation suggests that reduced insulin signaling ability by skeletal muscle, rather than visceral fat deposition or pancreatic failure, plays a central role in post-irradiation diabetogenesis.[43] Tonorezos et al have surmised that as visceral adiposity increases with age, and the sarcopenic phenotype begins to predominate, survivors are no longer able to compensate for radiation-induced pancreatic insufficiency and thus ultimately fail to maintain glucose homeostasis.[10] Further work is needed to clarify how the interplay of these factors impacts the pathophysiology of DM development in survivors of childhood cancer over time.
Poor dietary habits and physical activity limitations
Dietary habits and physical activity have a robust effect on metabolism and cardiovascular health. In the general population, many studies have proven the association of a healthy lifestyle with reduction in prevalence of insulin resistance, hypertension, and dyslipidemia.[44,45] In addition, a healthy diet with regular physical activity reduces the risk of cardiovascular morbidity and mortality, as well as cancer risk, among other clinical outcomes.
There is a growing body of literature describing poor adherence to dietary guidelines and a relatively sedentary lifestyle in survivors of childhood cancer. In 2007, Robien and colleagues assessed dietary intake of adult survivors of pediatric ALL using the National Cancer Institute Diet History Questionnaire.[46] Adherence to three commonly used recommendations, namely the Dietary Approaches to Stop Hypertension (DASH) diet, the US Department of Agriculture Food Guide, and the World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention guide, was assessed. Survivors showed low scores on dietary adherence to nutrition guidelines, mainly low fiber consumption and high sodium, added sugar, and meat consumption. A recent study of 413 adult survivors of childhood cancer and 361 controls that assessed both nutrition and physical activity resulted in similar findings.[47] Interestingly, there was no difference between survivors and controls regarding adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity. Both survivors and controls scored poorly on adherence to dietary recommendations. Survivors had significantly lower fiber consumption compared with controls (only 10% consuming adequate amounts).
In a 2013 paper, Tonorezos and colleagues linked adherence to the Mediterranean diet pattern (consisting of high intake of fruit, vegetables, legumes, and fish; moderate alcohol intake; and low intake of dairy and meat) with metabolic traits in adult childhood ALL survivors.[48] Adherence to the Mediterranean diet was associated with a reduced risk for metabolic syndrome, lower visceral and subcutaneous fat, and lower BMI. Surprisingly, even small changes in adherence substantially appeared to affect metabolism. They observed a 31% decrease in risk for metabolic syndrome with even one point increase of the diet score (for example, eating an additional half serving of vegetables per day).
Physical activity levels in survivors correspond to cardiovascular risk factor prevalence and, even more importantly, to risk of cardiovascular events.[49,50] For example, Hodgkin lymphoma survivors who reported vigorous exercise were 51% less likely to have a cardiovascular event.[49] In the study previously noted by Berdan et al, which was questionnaire-based, 77.4% of survivors met physical activity recommendations.[47] However, in other studies, physical activity levels were much lower. Most studies show that adult survivors of childhood cancer are significantly less physically active compared with healthy controls.[51,52] For example, in the Robien et al study, only 18% of survivors reported exercising for 30 minutes, 5 days a week.[46]
Many factors affect the manner in which pediatric cancer patients eat and exercise, including habits formed during cancer treatment. Familial concern over weight loss during treatment, concomitant corticosteroid use, neurotoxic agents, and avoidance of physical activity due to reduced muscle mass and fear all play a role in the difficulty of maintaining a healthy lifestyle after cancer treatment is over.[53,54] Also, some survivors have physical and cognitive limitations that prevent them from achieving desirable physical activity levels, such as patients who had brain tumors and those with limb amputations and joint replacements.[55-57]
Changes to gut microbiota
In recent years, it has been suggested that under some circumstances, such as a fat-enriched diet, modifications to the gut microbiota result in metabolic derangements such as insulin resistance, steatosis of the liver, and obesity. This is attributed both to enhanced energy harvest by the microbiota, allowing more calories to be digested, and to alterations to the immune system, causing a chronic inflammatory state that is characterized by elevated levels of interleukin (IL)-6, IL-1, and tumor necrosis factor-α, possibly influencing insulin signaling.[58-62] For example, artificial sweeteners seem to have this effect by changing the composition of gut microbiota, thus inducing glucose intolerance.[63] Although further research is needed, alterations in intestinal microbiota that occur during treatment could be expected to impact long-term metabolic outcomes. For example, there is evidence that chemotherapy changes the composition of intestinal microbiota, although whether these changes have long-term metabolic sequelae is unknown.[64-66]
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"43368","attributes":{"alt":"","class":"media-image","id":"media_crop_304473725612","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"4724","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","title":"","typeof":"foaf:Image"}}]]
Smita Bhatia, MD, MPH
Institute for Cancer Outcomes and Survivorship
University of Alabama at Birmingham, Birmingham, AlabamaWhat Have Been the Key Issues in This Area?
Obesity, insulin resistance/diabetes mellitus, hypertension, and dyslipidemia are a well-described set of long-term complications in childhood cancer survivors, and increase the risk for subsequent development of cardiovascular disease. Cranial radiation at a young age in girls increases risk of obesity, and total body and abdominal irradiation increase the risk of insulin resistance and dyslipidemia. Sarcopenia may aggravate the situation, as do poor diet and physical inactivity. The role of gut microbiota is currently under investigation. The few intervention studies that have been completed to date have shown a modest impact on obesity and cardiometabolic profile. This review does a great job in synthesizing the evidence to provide a balanced overview of the problem related to the cardiometabolic pathology faced by childhood cancer survivors, the extant literature on the pathogenesis, and the current evidence regarding the efficacy of the intervention strategies.What Issues Still Need to Be Addressed?
Increasing awareness regarding the magnitude of this problem, highlighting the vulnerable populations, and describing the current literature on the interventions that have demonstrated efficacy are needed-such that the clinicians can recognize this issue and address it appropriately when seeing the childhood cancer survivor in their practice. The issues that have yet to be clarified include the predilection for the development of obesity among girls exposed to cranial radiation at a young age; as well as the molecular pathogenesis of cardiometabolic perturbations, especially as the molecular underpinnings interact with therapeutic exposures and lifestyle behaviors. It is important to understand when to intervene and how to intervene, so that the interventions are effective. Finally, it is important to see how the changes in therapy will modify this cardiometabolic pathology.What Can We Expect in the Future?
We can expect that we will have developed a prediction model in which we would be able to anticipate and predict those childhood cancer survivors who are at highest risk of developing cardiopulmonary perturbations, which would allow for targeted interventions.
Intervention Studies
The mainstay of intervention for obesity and metabolic disease in the general population is diet and exercise. As previously noted, some groups of childhood cancer survivors are at high risk for obesity and metabolic disease.[15,21] Still, there have only been a handful of studies to assess the effectiveness of lifestyle interventions on the childhood cancer survivor population.
A study by Jarvela et al assessed the efficacy of a 16-week, home-based exercise program for long-term survivors of ALL.[67] The 17 patients studied were between the ages of 16 and 30, with all participants more than 10 years from diagnosis. Outcomes assessed included cardiometabolic risk factors (fasting insulin, HOMA-IR, fasting glucose, lipid profiles, and systolic blood pressure), with a significant improvement in fasting insulin, HOMA-IR, and systolic blood pressure at the end of the study period. Additionally, there was a significant decrease in waist circumference, waist-to-hip ration, and percentage of fat. There was no change in weight or BMI.[67]
Physical exercise interventions during or immediately following treatment for childhood cancer have also been studied. A Cochrane systematic review by Braam et al[68] assessed physical exercise training studies conducted within the first 5 years following a diagnosis of cancer. Five studies were identified, which included 131 children with ALL. Each of the studies compared a home-based exercise program with usual care, although the characteristics of the exercise program varied between studies. Primary outcomes included cardiorespiratory fitness, muscle endurance/strength, body composition, flexibility, activity energy expenditure, level of daily activity, and time spent exercising. Secondary outcomes included health-related quality of life, fatigue, anxiety and depression, self-efficacy, and adverse effects. Despite limitations in the studies due to small samples and insufficient study methodology, exercise was found to trend towards better physical fitness in the exercise intervention group, with statistical significance observed in some measures of cardiorespiratory fitness and flexibility. Importantly, only two of the five studies included BMI as an outcome, and results were not significant.[68]
Because of growing interest in the effectiveness of diet and exercise on cardiometabolic risk modification in the childhood cancer survivor population, a small number of National Institutes of Health–supported studies are looking to provide insight into effective interventions. For example, Let’s Play! Healthy Kids After Cancer is a nutrition and physical activity intervention targeted at the parents of young ALL survivors (age 4–10 years old). The study aims to modify the home environment to improve physical activity and diet, so as to mitigate unhealthy weight gain after chemotherapy. At the end of the 6-month intervention period, changes in weight-related behaviors will be noted, as will the impact of the intervention on biomarkers of inflammation and oxidative stress, fatigue, and body composition (ClinicalTrials.gov identifier: NCT02361047).
Exercise and Quality Diet After Leukemia (EQUAL) is an ongoing diet and physical activity intervention aimed at long-term adult survivors of childhood ALL. The EQUAL study will compare survivors randomized to a diet and physical activity intervention, delivered via telephone and Web support, with survivors given printed materials about healthy habits. As an ancillary study of the CCSS, the EQUAL study includes participants from all over the United States and should, once results are available, provide important information regarding potential interventions for this population (ClinicalTrials.gov identifier: NCT02244411).
Conclusions
Metabolic syndrome, DM, and obesity are, unfortunately, common and significant late effects of childhood cancer. In particular, survivors of ALL and survivors treated with cranial, total body, or abdominal radiation are at higher risk for these outcomes. As a result, these survivors are at increased risk for premature cardiovascular disease. Additionally, with the general population becoming increasingly obese, recognizing metabolic disease and its complications is essential to improving the care of the childhood cancer survivor.
Potential mechanisms for the observed increase in risk include alterations in leptin and adiponectin, pancreatic insufficiency, poor dietary habits, sedentary lifestyle, and perhaps changes in the composition of the gut microbiota, many of which could be the consequence of RT. Despite advances in our understanding of the altered metabolism after childhood cancer, the exact mechanisms involved are still poorly understood.
Thus far, exercise intervention studies in childhood cancer survivors have shown moderate improvements in some metabolic parameters. The ongoing studies detailed in this review are the first steps to defining the appropriate diet and exercise prescription for the at-risk childhood cancer survivor. Investigation of the mechanism of metabolic disease allows for targeted therapy down the road; it also may help modify therapeutic regimens during cancer treatment itself. Further research is warranted to evaluate the barriers to a healthy lifestyle and other interventions in childhood cancer survivors.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Note: Drs. Barnea and Raghunathan contributed equally to the manuscript.
Acknowledgment:This work was supported by the National Institutes of Health (R01-CA187397), the American Institute of Cancer Research, and grant KL2RR000458 of the Clinical and Translational Science Center at Weill Cornell Medical College.
References:
1. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute. Bethesda, Maryland; 2013.
2. Smith MA, Altekruse SF, Adamson PC, et al. Declining childhood and adolescent cancer mortality. Cancer. 2014;120:2497-506.
3. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61-70.
4. Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334-43.
5. Kersey JH. Fifty years of studies of the biology and therapy of childhood leukemia. Blood. 1997;90:4243-51.
6. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-82.
7. Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371-81.
8. Armstrong GT, Pan Z, Ness KK, et al. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28:1224-31.
9. Yeh JM, Nekhlyudov L, Goldie SJ, et al. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152:409-17, W131-8.
10. Tonorezos ES, Hudson MM, Edgar AB, et al. Screening and management of adverse endocrine outcomes in adult survivors of childhood and adolescent cancer. Lancet Diabetes Endocrinol. 2015;3:545-55.
11. Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186-95.
12. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
13. Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033-40.
14. Siddiqui AQ, Howlader MS, Adam AA. Effects of dietary-protein levels on growth, feed conversion and protein-utilization in fry and young Nile tilapia, Oreochromis-niloticus. Aquaculture. 1988;70:63-73.
15. Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313-20.
16. Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170-81.
17. Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698-704.
18. Janiszewski PM, Oeffinger KC, Church TS, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92:3816-21.
19. Malhotra J, Tonorezos ES, Rozenberg M, et al. Atherogenic low density lipoprotein phenotype in long-term survivors of childhood acute lymphoblastic leukemia. J Lipid Res. 2012;53:2747-54.
20. Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359-65.
21. Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169:1381-8.
22. Zhang FF, Liu S, Chung M, Kelly MJ. Growth patterns during and after treatment in patients with pediatric ALL: a meta-analysis. Pediatr Blood Cancer. 2015;62:1452-60.
23. Lorini R, Cortona L, Scaramuzza A, et al. Hyperinsulinemia in children and adolescents after bone marrow transplantation. Bone Marrow Transplant. 1995;15:873-7.
24. Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765-72.
25. Oudin C, Auquier P, Bertrand Y, et al. Metabolic syndrome in adults who received hematopoietic stem cell transplantation for acute childhood leukemia: an LEA study. Bone Marrow Transplant. 2015 Jul 20. [Epub ahead of print]
26. Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117:4442-8.
27. Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16:1674-81.
28. Hoffmeister PA, Hingorani SR, Storer BE, et al. Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:515-24.
29. Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:619-25.
30. Teinturier C, Tournade M-F, Caillat-Zucman S, et al. Diabetes mellitus after abdominal radiation therapy. Lancet. 1995;346:633-4.
31. Cicognani A, Cacciari E, Mancini AF, et al. Abnormal insulin response to glucose following treatment for Wilms’ tumor in childhood. Eur J Pediatr. 1997;156:371-5.
32. Hawkins MM, Robertson CM, Edge JA, Neil HAW. Is risk of diabetes mellitus increased after abdominal radiotherapy? Lancet. 1996;347:538-40.
33. Holmqvist AS, Olsen JH, Andersen KK, et al. Adult life after childhood cancer in Scandinavia: diabetes mellitus following treatment for cancer in childhood. Eur J Cancer. 2014;50:1169-75.
34. de Vathaire F, El-Fayech C, Ben Ayed FF, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol. 2012;13:1002-10.
35. van Nimwegen FA, Schaapveld M, Janus CP, et al. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma. J Clin Oncol. 2014;32:3257-63.
36. Tonorezos ES, Vega GL, Sklar CA, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer. 2012;58:31-6.
37. Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:3558-62.
38. Du Toit DF, Heydenrych JJ, Smit B, et al. The effect of ionizing radiation on the primate pancreas: an endocrine and morphologic study. J Surg Oncol. 1987;34:43-52.
39. Sarri Y, Conill C, Verger E, et al. Effects of single dose irradiation on pancreatic beta-cell function. Radiother Oncol. 1991;22:143-4.
40. Ahmadu-Suka F, Gillette EL, Withrow SJ, et al. Pathologic response of the pancreas and duodenum to experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys. 1988;14:1197-204.
41. Heijmans HJ, Mehta DM, Kleibeuker JH, et al. Intraoperative irradiation of the canine pancreas: short-term effects. Radiother Oncol. 1993;29:347-51.
42. Oeffinger KC, Sklar CA. Abdominal radiation and diabetes: one more piece in the puzzle. Lancet Oncol. 2012;13:961-2.
43. Kavanagh K, Dendinger MD, Davis AT, et al. Type 2 diabetes is a delayed late effect of whole-body irradiation in nonhuman primates. Radiat Res. 2015;183:398-406.
44. Kastorini CM, Milionis HJ, Esposito K, et al. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299-313.
45. Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4. pii: e002014.
46. Robien K, Ness KK, Klesges LM, et al. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:815-22.
47. Berdan CA, Tangney CC, Scala C, Stolley M. Childhood cancer survivors and adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity. J Cancer Surviv. 2014;8:671-9.
48. Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24:313-21.
49. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32:3643-50.
50. Slater ME, Ross JA, Kelly AS, et al. Physical activity and cardiovascular risk factors in childhood cancer survivors. Pediatr Blood Cancer. 2014 Oct 18. [Epub ahead of print]
51. Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: a review of the literature. Ann Behav Med. 2010;39:232-49.
52. Hocking MC, Schwartz LA, Hobbie WL, et al. Prospectively examining physical activity in young adult survivors of childhood cancer and healthy controls. Pediatr Blood Cancer. 2013;60:309-15.
53. Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975-81.
54. Fuemmeler BF, Pendzich MK, Clark K, et al. Diet, physical activity, and body composition changes during the first year of treatment for childhood acute leukemia and lymphoma. J Pediatr Hematol Oncol. 2013;35:437-43.
55. Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116:3034-44.
56. Wampler MA, Galantino ML, Huang S, et al. Physical activity among adult survivors of childhood lower-extremity sarcoma. J Cancer Surviv. 2012;6:45-53.
57. Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984-94.
58. Burcelin R, Garidou L, Pomie C. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol. 2012;24:67-74.
59. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-6.
60. Lankelma JM, Nieuwdorp M, de Vos WM, Wiersinga WJ. The gut microbiota in internal medicine: implications for health and disease. Neth J Med. 2015;73:61-8.
61. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-4.
62. Vrieze A, Out C, Fuentes S, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824-31.
63. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-6.
64. Touchefeu Y, Montassier E, Nieman K, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409-21.
65. van Vliet MJ, Tissing WJ, Dun CA, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49:262-70.
66. Zwielehner J, Lassl C, Hippe B, et al. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One. 2011;6:e28654.
67. Jarvela LS, Kemppainen J, Niinikoski H, et al. Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:155-60.
68. Braam KI, van der Torre P, Takken T, et al. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2013;4:Cd008796.