Olaparib Looks Promising in Treatment of Non-BRCA Ovarian Cancer
Researchers at the BC Cancer Agency in Vancouver and colleagues have just published the results of a phase II study showing that olaparib (AZD2281), an oral PARP inhibitor, may be effective in treating non-BRCA-related ovarian cancer patients.
Researchers at the BC Cancer Agency in Vancouver and colleagues have just published the results of a phase II study showing that olaparib (AZD2281), an oral PARP inhibitor, may be effective in treating non-BRCA–related ovarian cancer patients. The research was published in the Lancet Oncology on August 22, 2011 ( DOI: 10.1016/S1470-2045(11)70214-5).
The Background
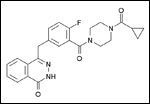
Chemical structure of olaparib
It’s widely understood that carriers of mutations in BRCA1 and BRCA2 carriers are at elevated risk of cancer; by age 70 the risk of breast cancer in this group is 50% to 87% greater, and the risk of ovarian cancer is 10% to 40% greater. Additionally, women with these mutations often present with ovarian cancer at a later disease stage-more than 70% present at stage III or IV.
Olaparib has previously been shown effective in a phase I study with an expanded cohort of BRCA1 or BRCA2 carriers with ovarian cancer-follow-up phase II studies also confirmed the effectiveness of olaparib, with response rates of 41% (11 of 27 patients) in a group of patients with advanced breast cancer and 33% (11 of 33 patients) in a group of patients with ovarian cancer.
The Study
The phase II study conducted by Karen Gelmon, MD and her colleagues was multicenter and non-randomized, and enrolled women with advanced, high-grade serous and/or undifferentiated ovarian cancer or triple-negative breast cancer (TNBC), with or without the BRCA1 or BRCA2 mutation. (Patients with the BRCA1 or BRCA2 mutations were enrolled for comparison-the results of the studies with olaparib on patients with ovarian and breast cancers carrying the BRCA1 or BRCA2 mutations were not known at the inception of this trial.)
The study had four cohorts: Group A consisted of patients with TNBC and either negative or unknown BRCA1 or BRCA2 mutations. Group B included patients with advanced, recurrent breast cancer with known BRCA1 or BRCA2 mutations. Group C had patients with recurrent ovarian cancer and known BRCA1 or BRCA2 mutations, and Group D had patients with advanced recurrent ovarian cancer with negative BRCA1 or BRCA2 status.
All patients received 400mg of olaparib twice a day on a continuous basis, and the primary endpoint was objective response rate by Response Evaluation Criteria In Solid Tumors (RECIST).
The Results
In total, 91 patients were enrolled in the four cohorts; 65 who had ovarian cancer and 26 who had breast cancer. Of the 91 patients, 90 were treated in the time span between July 2008 and September 2009. In the 63 patients with ovarian cancer who were eligible for objective response due to RECIST, confirmed response was seen in 7 of the 17 patients with BRCA1 or BRCA2 mutations and 11 of the 46 who did not have these mutations. However, no objective responses were seen in any of the breast cancer patients. The most common side effects were fatigue, nausea and vomiting, and decreased appetite.
Conclusion
The study shows that olaparib is promising for women with ovarian cancer, but further clinical trials of the agent are needed.