Radiation therapy continues to be a key component in the management of pediatric malignancies. Increasing the likelihood of cure while minimizing late treatment toxicity in these young patients remains the primary goal. Within the realm of central nervous system neoplasms, efforts to further improve the efficacy of radiation therapy continue, while balancing risks of damage to uninvolved tissue. Radiation therapy can result in second malignancies, as well as cerebrovascular, neurotoxic, neurocognitive, endocrine, psychosocial, and quality-of-life effects. In this article we describe these acute and late effects and their implications, and we highlight strategies that have emerged to reduce both the volume of tissue that is irradiated and the radiation dose delivered. The feasibility, efficacy, and risks of these newer approaches to radiation therapy continue to be evaluated and monitored; robust outcome data are needed.
Introduction
Radiation therapy is a major treatment avenue for the management of pediatric central nervous system (CNS) tumors. Efforts are ongoing to further improve the efficacy of this treatment modality, with the goal of curing pediatric cancers. Minimizing late treatment toxicities in these patients remains an important priority due to both the young age of the patients and the high cure rate that can be achieved. A better understanding of the mechanisms underlying normal tissue injury is essential to preventing or reducing the deleterious effects of treatment. While novel radiation techniques are currently underinvestigated in the pediatric population, their feasibility, efficacy, and risks continue to be evaluated and monitored. In an effort to improve patient outcomes, clinicians are employing advanced surgical methods, increased use of systemic agents, increased use of high-precision imaging, and strategies to decrease the frequency of imaging, in combination with the newer radiation techniques.
Complications of Therapy
Naturally, the long-term effects of radiation therapy are of great importance in the pediatric population. As treatment advances enable patients with certain pediatric malignancies to live longer, the toxicities of oncologic therapies administered early in life may become clinically apparent years, or even decades, later. With the advent of modern technology, survival rates have been increasing: more than 80% of all pediatric cancer patients, and in particular 70% of children with primary CNS cancers, are surviving beyond 5 years.[1] It has been estimated that by 2020 there will be more than 500,000 survivors of pediatric malignancy.[2]
Primary CNS tumors in children encompass a broad spectrum of pathologies, ranging from histologically benign but functionally detrimental craniopharyngiomas, to malignant high-grade gliomas with poor prognosis. The Childhood Cancer Survivor Study (CCSS) is one of the largest cohorts of childhood cancer survivors available from which the late effects of pediatric cancer therapy can be ascertained. The year of initial diagnosis in these patients dates from the 1970s to the present, and represents the changes in treatment paradigms and technology that have occurred over the past 45 years. Results from the CCSS show a decline in treatment-related toxicity, and suggest that methods to reduce late morbidity have been effective at extending the life-spans of children treated for cancer.[3]
Radiation therapy is known to contribute to late morbidity and mortality in the pediatric population, and complications can present in the acute, subacute, and late term. The manifestation of these effects depends on the location of the irradiated tumor and the age at which the patient underwent radiation therapy. Radiation therapy to the craniospinal axis can lead to cerebrovascular disease, neurotoxicity, neurocognitive decline, development of second malignancies, endocrine deficits, negative quality-of-life effects, and psychosocial late effects.
Cerebrovascular effects
Radiation-induced cerebrovascular disease can manifest as intracranial steno-occlusive disease, microangiopathy, vascular malformations, intracranial aneurysm, moyamoya disease, and internal carotid artery stenosis. Damage induced by radiation in the acute phase is primarily manifested in the small arteries and arterioles. However, as late changes occur, increased damage is seen in the intermediate-sized and larger arteries, presumably as a result of ongoing inflammatory and oxidative stress.[4]
Data regarding the risk of late cerebrovascular damage are lacking. In the CCSS, the risk of cerebrovascular accident was more than 10 times higher for cancer survivors than for their sibling controls. The mean interval from cancer diagnosis to late-occurring stroke was 13.9 years (standard deviation, 6.3 years), and a dose-dependent relationship was observed, with cranial radiation doses ≥ 50 Gy being 3.3 times more likely to be associated with late-occurring stroke.[5]
Neurotoxicity and neurocognitive effects
The CNS, and in particular the brain, is exquisitely sensitive to insults as it continues to develop and mature throughout childhood. Damage to the brain parenchyma can manifest as radiation necrosis, occurring in 4.6% of patients at a median of 5 months posttreatment (range, 0 to 131 months).[6,7] The exact definition of radiation necrosis is somewhat heterogeneous in the clinical literature, ranging from asymptomatic imaging changes to symptomatic disability or pathologically positive necrosis on rebiopsy. Symptoms of radiation necrosis can include ataxia, cranial nerve palsies, weakness, headache, or vision changes, depending on the location of the damage. Rates of radiation necrosis and late morbidity are therefore dependent on the frequency of routine imaging and clinical assessment, as well as on the ability to differentiate these radiation-induced changes from effects related to malignant progression or sequela from alternative treatment modalities.
The neurocognitive effects of radiation have been well documented in pediatric studies. Treatment with radiation has been reported to reduce Intelligence Quotient (IQ) scores, verbal skills, visual-spatial skills, attention, memory, psychomotor speed, executive function, processing speed and working memory, and academic achievement.[8,9] The etiology of neurocognitive sequelae of radiation therapy is complex; it may involve oxidative stress, inflammation, and hypoxic/ischemic injury, leading to apoptosis, endothelial dysfunction, inhibition of neurogenesis, demyelination, and tissue necrosis.[10]
Younger age at treatment, larger radiation field, higher radiation dose, longer time from treatment, implantation of a ventriculoperitoneal shunt, and female sex have been implicated as risk factors for neurocognitive impairment.[8] In addition, treatment-induced ototoxicity or ocular toxicity may compound neurocognitive deficits.[11] Pediatric patients have been found to be almost one standard deviation below normal with respect to cognitive ability, and their IQ scores have been shown to progressively decline by as much as 12 to 14 points from their pretreatment scores.[9] It has been theorized that, rather than reflecting a loss of knowledge, changes in IQ scores over time among pediatric cancer patients and survivors may be due to a decline in the ability to gain new information, in comparison to the general population, with a significant age effect noted.[8,11]
Second malignancy
Second tumors are rare complications of radiation therapy, but are noteworthy in the pediatric population due to potential longevity of the patient posttreatment. Second neoplasms have shown linear dose-response rates, with a 3.18% risk at 20 years for all pediatric cancers, and a 2.14% risk for patients irradiated for pediatric CNS tumors. Latency of second neoplasms depends on the type of cancer, with new leukemia peaking at 5 to 7 years, and new solid tumors developing 10 years or more posttreatment.[12,13]
Causality in second cancers can be difficult to elucidate, since many patients are exposed to multiple treatment modalities, have differing environmental and host factors, and may have a genetic predisposition to malignancy. An analysis from a Surveillance, Epidemiology, and End Results Program cohort of adult patients 20 or more years after diagnosis of malignancy in childhood or adolescence estimated that only 8% of solid second cancers reported were directly attributable to the primary radiation field, with approximately 5 excess cancers occurring per 1,000 patients treated with radiation at 15 years after diagnosis.[14] Risk factors for second malignancy include younger age, larger radiation field size, conformity of radiation, increased amount of time since treatment, and risks vary based on the type of tissue exposed.[12,14] The most common second malignancy in patients who receive radiation to the brain is meningioma, which usually occurs 20 or more years after primary treatment.
Endocrine effects
Late endocrine effects are relatively common in survivors of pediatric malignancy. The incidence rate of endocrinopathies in pediatric patients treated for CNS tumors is documented to be between 43% and 80%. Endocrine dysfunction can present in a variety of ways related to hormone dysregulation-such as growth delay, hyperprolactinemia, central hypothyroidism, central adrenal insufficiency, precocious puberty, low bone mineral density, and metabolic syndrome.[15]
Growth hormone deficiency is the most common effect of radiation therapy, followed by hypothyroidism. Growth hormone deficiencies can result from radiation doses as low as 18 Gy to the hypothalamus, whereas other manifestations of endocrine dysfunction usually become clinically apparent at doses of 40 Gy to 50 Gy.[15] Risk factors for hypothalamic-pituitary dysfunction include younger age, larger area irradiated, higher total dose, higher dose per fraction, and a longer period of time since treatment.[15,16] Abnormalities in normal endocrine function can result in late sequelae that compound other late effects of treatment. For example, metabolic syndrome due to pituitary axis dysfunction can further exacerbate vascular disease or alter second malignancy risks.
Psychosocial effects and quality of life
Pediatric cancer survivors may experience many negative effects on long-term health-related quality of life (HRQOL), as a result of their underlying malignancies and the treatment-related toxicities mentioned previously. One of the earliest analyses of self-esteem among pediatric cancer survivors demonstrated significantly lower self-esteem compared with normal controls globally, and specifically in the domains of peer interaction, work, body image, and sports/physical activity.[17]
The CCSS cohort has demonstrated functional limitations in physical performance and tasks of daily living. Nonwhite ethnicity and disfigurement were associated with poor mental HRQOL. Disfigurement and obesity, as well as female sex, older current age, and lower levels of education and household income, were associated with poor physical HRQOL.[18] A 2004 report by Zebrack et al noted that the variables leading to physiologic distress in pediatric cancer survivors were not specific to their treatment, but instead were shared with the general population; these included lower household income and educational attainment, being unmarried, not being employed in the past 12 months, and female sex.[19]
The CNS patients from the CCSS cohort were less likely to marry and more likely to divorce; and they had higher levels of unemployment and lower levels of education.[20,21] The CCSS cancer survivors were twice as likely to live dependently, compared with their sibling controls (17.7% vs 8.7%), and this was more common in patients with CNS tumors than in those who had experienced other pediatric malignancies.[22] Pediatric cancer survivors have also been reported to have higher rates of suicidal ideation, use of prescribed psychoactive substances, depression and anxiety, and antisocial behaviors.[23,24]
Strategies to Reduce the Toxicity of Radiation Therapy
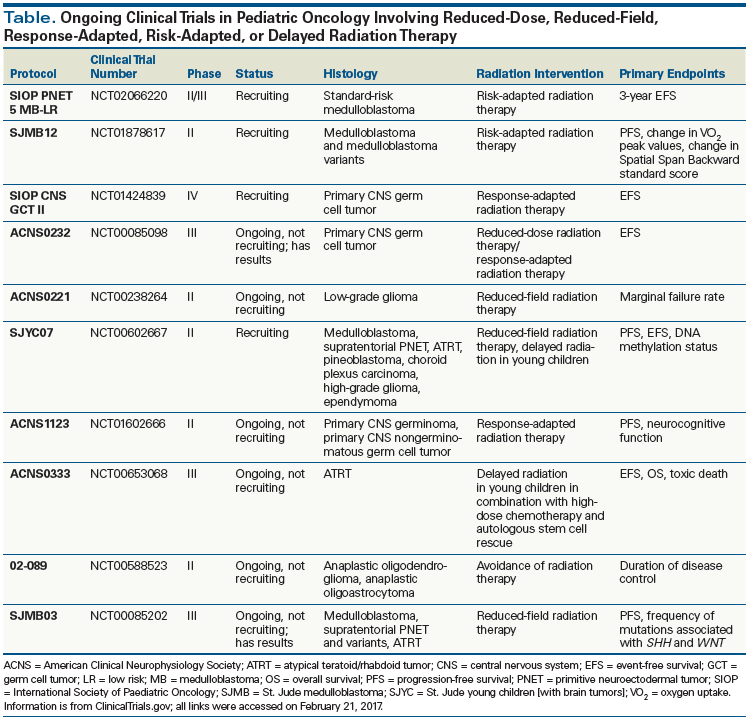
The significant morbidity experienced by some pediatric cancer survivors raises the question of how the therapeutic index can be altered to maximize outcomes. As imaging modalities, conformity of radiation techniques, chemotherapy options, and surgical techniques improve, strategies to delay or forgo radiation, as well as reduce radiation dose or treatment volumes, have become increasingly sought after and feasible (Table).
Timing of radiation
Epidemiologic studies of the effects of radiation on the pediatric brain have demonstrated a relationship between timing, dose, and volume of radiation therapy. The possibility of avoiding the need for radiation completely, or delaying its use in young children, has been an active area of research in the pediatric population. Multiple studies are evaluating the sequencing of radiation in the treatment paradigm, since alternative modalities may have improved efficacy when given before, after, or concurrently with radiation (see Table).
Younger age at time of treatment is a consistent risk factor for late sequelae of radiation toxicity, with one of the most notable age-response relationships evident in studies of neurocognitive effects.[8,25,26] For this reason, radiation oncologists have hesitated to use radiation in young children and infants. Response-based radiation has been employed in multiple protocols (see Table) in an attempt to control disease with surgery and/or chemotherapy first, or avoid radiation altogether until patients reach a specified age. An important key to these strategies is the ability to determine the age at which patients are most susceptible to radiation-induced toxicity. Therefore, continued study of the mechanisms underlying radiation damage is imperative in this population.
In order to reduce radiation-related toxicity, the Head Start III trial attempted to defer radiation in young children with ependymoma using dose-intense chemotherapy and autologous hematopoietic stem cell rescue. This proved to be an effective strategy in patients with supratentorial ependymomas, but was ineffective in patients with infratentorial disease.[27] There is, however, prospective evidence that radiation is safe and effective in very young patients, and that improved oncologic outcomes in ependymoma counterbalance toxicity, thus making radiation therapy necessary to achieving the goal of cure.[28,29] In the era of precision medicine, and with the adoption of molecular classifications of disease, personalized treatment based on biology, age, and disease response may become a feasible and appropriate future approach.[30-32] However, if delaying or avoiding irradiation in an individual patient would not result in superior outcomes, then other methods to reduce toxicity while maintaining the role of radiation need to be pursued.
Reduction in radiation dose
Radiation dose has also been correlated with risk of toxicity for certain late effects in pediatric malignancies. Analysis of the CCSS cohort suggests that the risk of stroke increases by 5% per Gy of radiation delivered (with an overall rate of first stroke reported as 625 per 100,000 person-years),[33] and excess relative risk for a second CNS malignancy is 0.69 per Gy of radiation exposure.[34] For this reason, efforts are ongoing to minimize the total dose delivered to pediatric patients; dose de-escalation may be a valid method to reduce late morbidity, particularly in patients whose cancers carry an excellent prognosis.
In most cases, dose de-escalation is made possible via synergy with other treatment modalities to maintain the desired clinical outcomes. The evolution of therapeutic approaches for management of medulloblastoma demonstrates this paradigm. In recent decades, the administration of chemotherapy enabled successful reduction of the dose of craniospinal irradiation (CSI) required to treat standard-risk medulloblastoma-from 36 Gy to 23.4 Gy, followed by a posterior fossa boost.[35] However, trials of radiation dose de-escalation in the absence of chemotherapy showed inferior survival outcomes.[36,37] With this change in dose to the spinal axis, multiple groups have demonstrated improved neurocognitive outcomes in the 23.4-Gy CSI cohort.[25] These findings precipitated further reduction of the CSI dose to 18 Gy in young patients; however, recent data suggest that reducing the radiation dose to this level is associated with inferior event-free survival and overall survival outcomes.[38]
Since advances in surgical and systemic therapy can reduce the burden of disease, response-based dose de-escalation of radiation therapy is being investigated to provide individualized treatment options. As an example, retrospective and early-phase trials including patients with intracranial germ cell tumors report equivalent outcomes with response-based radiation therapy, and these results have provided the basis for several ongoing phase III trials (see Table).
Reduction in treatment volumes
Increasing the conformity of treatment in order to reduce the volume of normal tissue exposed to radiation is an additional way to reduce late toxicity. There are two relevant components: the conformity of the high-dose region to the treatment target; and the overall volume of tissue irradiated, even to a low dose.
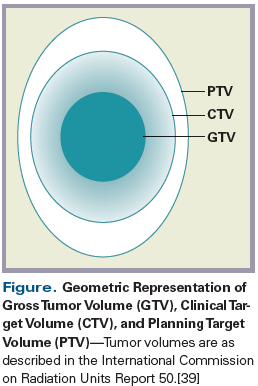
Radiation volumes are outlined based on the visible tumor seen on imaging and during physical examination (gross tumor volume; the area encompassing subclinical disease, based on known patterns of spread (clinical target volume [CTV]); and a margin of error for treatment uncertainties (planning target volume) (Figure).[39] Minimizing these volumes, or increasing the accuracy of dose delivery, can have a substantial impact on the total amount of tissue treated-just as the difference in the total volume of a sphere can increase dramatically when the radius is increased even as little as a few millimeters.
KEY POINTS
- Radiation is one of the most effective therapies to date for many pediatric malignancies, but exposure of the developing brain to radiation can be associated with cerebrovascular events, secondary malignancy, endocrine effects, and psychosocial/quality-of-life disturbances.
- Numerous clinical trials now underway are investigating reduced-dose, reduced-field, response-adapted, risk-adapted, or delayed radiation therapy to better understand which approaches will have the most meaningful impact with the least possible toxicity.
- Accurate imaging is the cornerstone of tumor identification, target volume delineation, and radiation treatment planning; modalities most commonly used in the pediatric population include CT, MRI, and functional imaging.
Accurate imaging is the cornerstone of tumor identification, target volume delineation, and radiation treatment planning. Modalities that are most commonly used in the pediatric population include CT, MRI, and functional imaging. In recent years the wise philosophy of modifying imaging techniques in order to reduce unnecessary toxicity from diagnostics has taken hold. The use of CT in the pediatric population is somewhat discouraged, since CT delivers ionizing radiation and has inferior soft-tissue resolution compared with MRI. There is, however, ongoing research in the pediatric population to develop novel CT imaging methods that minimize the radiation dose delivered to these young patients.[40]
The use of MRI has become more ubiquitous in the imaging of primary CNS diseases, due to its benefits of reduced toxicity (since it provides nonionizing radiation) and superior multifaceted soft-tissue resolution compared with CT. The gross tumor volume can be optimized with modern MRI-based imaging techniques, resulting in excellent gross tumor delineation. Novel MRI sequences, such as diffusion- and perfusion-weighted imaging, are currently under investigation for use in differentiating tumor grade in situ, predicting pathways of microscopic spread, and monitoring treatment response.[41,42] Knowledge of patterns of spread in situ for individual patients will allow radiation oncologists to more accurately conform CTV to true subclinical disease and avoid toxicity to uninvolved areas. In addition to these imaging advances, as we learn more about the mechanisms underlying late radiation treatment toxicity, therapy may be conformed away from uninvolved critical structures, such as the temporal lobes.[43]
Additional data can be gleaned from metabolic characterization of disease in situ. Fluorodeoxyglucose–positron emission tomography scanning has been applied in the pediatric population to aid in surgical resection, radiation treatment planning, and diagnosis of recurrent or residual disease.[44] Novel radiotracers, such as [11C]-methionine, [11C]-thymidine, [18F]-L-dihydroxyphenylalanine, and [18F]-fluorothymidine, characterize additional tumor metabolic pathways and may be useful as biomarkers for future radiation and surgical treatment planning.[44,45]
Newer surgical techniques and chemotherapeutic options may permit further cytoreduction of gross and subclinical disease, and may minimize the area requiring radiation. Modeling of the radiation dose to critical organs has shown a theoretical decrease in the risk of late toxicity when smaller treatment volumes are used.[43] In a prospective cohort of ependymoma, disease control was maintained when CTV volumes were decreased to 1 cm. The authors of this study highlight the benefits of neoadjuvant chemotherapy, as well as maximum safe resection, even if achieving this requires a second surgery.[28] However, it appears that for certain pathologies, reduced margins do not increase marginal relapse rates.[28,29,46] The late HRQOL and toxicity data from these reports will be of great interest as these cohorts mature.
Although not within the scope of this article, it is important to make note of novel systemic therapies under investigation for CNS diseases-including immunotherapies and targeted agents-which may further alter the therapeutic index.[47] These novel modalities may offer new opportunities to delay or forgo radiation therapy, and may present unique interactions when combined with radiation. Ultimately, the benefits of reduced radiation will need to be weighed against the potential risks of poorer survival outcomes, and long-term follow-up will be essential.
Conclusion
Diligent postradiation follow-up of pediatric patients is necessary for documentation, treatment, and ultimately prevention of late sequelae. Ongoing studies examining the efficacy of novel radiation therapy techniques will enable superior risk-benefit discussions. Improving the therapeutic index and maximizing outcomes for pediatric cancer patients is a rapidly evolving area of research, and will continue to be a multidisciplinary endeavor. The ongoing research into molecular classification of CNS tumors will result in new risk stratification of these diseases.[32] Given the diverse natural history of these subgroups, it will be important to revisit options for individual patients in terms of surgery, systemic therapy, and radiation techniques and agents. Notably, while alternative treatment modalities may be useful in reducing radiation toxicity, their long-term consequences must be studied with the same rigor that is applied to evaluation of radiation therapy, in order to more clearly understand the true risk-benefit effects.
Acknowledgment:This research was supported in part by the intramural research program at the National Institutes of Health, National Cancer Institute, Radiation Oncology Branch, funded by grant ZID BC 010990.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2011. http://seer.cancer.gov/csr/1975_2011/. Accessed February 9, 2017.
2. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61-70.
3. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833-42.
4. Morris B, Partap S, Yeom K, et al. Cerebrovascular disease in childhood cancer survivors: a Children’s Oncology Group report. Neurology. 2009;73:1906-13.
5. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277-82.
6. Drezner N, Hardy KK, Wells E, et al. Treatment of pediatric cerebral radiation necrosis: a systematic review. J Neurooncol. 2016;130:141-8.
7. Kralik SF, Ho CY, Finke W, et al. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am J Neuroradiol. 2015;36:1572-8.
8. Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705-17.
9. Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. 2016;31:237-54.
10. Fike JR. Physiopathology of radiation-induced neurotoxicity. Rev Neurol (Paris). 2011;167:746-50.
11. Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:3255-61.
12. Inskip PD, Sigurdson AJ, Veiga L, et al. Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys. 2016;94:800-7.
13. Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618-29.
14. Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224-33.
15. Follin C, Erfurth EM. Long-term effect of cranial radiotherapy on pituitary-hypothalamus area in childhood acute lymphoblastic leukemia survivors. Curr Treat Options Oncol. 2016;17:50.
16. van Waas M, Neggers SJ, Uitterlinden AG, et al. Treatment factors rather than genetic variation determine metabolic syndrome in childhood cancer survivors. Eur J Cancer. 2013;49:668-75.
17. Hornquist L, Rickardsson J, Lannering B, et al. Altered self-perception in adult survivors treated for a CNS tumor in childhood or adolescence: population-based outcomes compared with the general population. Neuro Oncol. 2015;17:733-40.
18. Nolan VG, Krull KR, Gurney JG, et al. Predictors of future health-related quality of life in survivors of adolescent cancer. Pediatr Blood Cancer. 2014;61:1891-4.
19. Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:999-1006.
20. Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2390-5.
21. Kirchhoff AC, Leisenring W, Krull KR, et al. Unemployment among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Med Care. 2010;48:1015-25.
22. Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1197-203.
23. Brinkman TM, Ullrich NJ, Zhang N, et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7:104-14.
24. Brinkman TM, Zhang N, Recklitis CJ, et al. Suicide ideation and associated mortality in adult survivors of childhood cancer. Cancer. 2014;120:271-7.
25. Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511-9.
26. Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55:525-31.
27. Venkatramani R, Ji L, Lasky J, et al. Outcome of infants and young children with newly diagnosed ependymoma treated on the “Head Start” III prospective clinical trial. J Neurooncol. 2013;113:285-91.
28. Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258-66.
29. Merchant TE, Bendel AE, Sabin N, et al. A phase II trial of conformal radiation therapy for pediatric patients with localized ependymoma, chemotherapy prior to second surgery for incompletely resected ependymoma and observation for completely resected, differentiated, supratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2015;93(suppl 3S):S1.
30. Strother DR, Lafay-Cousin L, Boyett JM, et al. Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: a report of the Pediatric Oncology Group randomized controlled trial 9233/34. Neuro Oncol. 2014;16:457-65.
31. Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8:696-705.
32. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-20.
33. Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:643-8.
34. Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528-37.
35. Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202-8.
36. Deutsch M, Thomas PR, Krischer J, et al. Results of a prospective randomized trial comparing standard dose neuraxis irradiation (3,600 cGy/20) with reduced neuraxis irradiation (2,340 cGy/13) in patients with low-stage medulloblastoma. A Combined Children’s Cancer Group–Pediatric Oncology Group study. Pediatr Neurosurg. 1996;24:167-76.
37. Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000;18:3004-11.
38. Michalski JM, Janss A, Vezina G, et al. Results of COG ACNS0331: a phase III trial of involved-field radiotherapy (IFRT) and low dose craniospinal irradiation (LD-CSI) with chemotherapy in average-risk medulloblastoma: a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2016;96:937-8.
39. Landberg T, Chavaudra J, Dobbs J, et al. Prescribing, recording, and reporting photon beam therapy (report 50). International Commission on Radiotherapy Units and Measurements. J ICRU. 1993.
40. Siegel MJ, Curtis WA, Ramirez-Giraldo JC. Effects of dual-energy technique on radiation exposure and image quality in pediatric body CT. AJR Am J Roentgenol. 2016 Aug 4. [Epub ahead of print]
41. Ho CY, Cardinal JS, Kamer AP, et al. Contrast leakage patterns from dynamic susceptibility contrast perfusion MRI in the grading of primary pediatric brain tumors. AJNR Am J Neuroradiol. 2016;37:544-51.
42. Marupudi NI, Altinok D, Goncalves L, et al. Apparent diffusion coefficient mapping in medulloblastoma predicts non-infiltrative surgical planes. Childs Nerv Syst. 2016;32:2183-7.
43. Brodin NP, Munck af Rosenschöld P, Blomstrand M, et al. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro Oncol. 2014;16:594-602.
44. Stanescu L, Ishak GE, Khanna PC, et al. FDG PET of the brain in pediatric patients: imaging spectrum with MR imaging correlation. Radiographics. 2013;33:1279-303.
45. Morana G, Piccardo A, Puntoni M, et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: a comparative study. Neuro Oncol. 2015;17:1637-47.
46. Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598-604.
47. Adamson PC. Improving the outcome for children with cancer: development of targeted new agents. CA Cancer J Clin. 2015;65:212-20.