Newer approaches in the field of radiation therapy have raised the bar in the treatment of central nervous system (CNS) malignancies, with recognized advances that have aimed to increase the therapeutic index by improving conformality of the radiation dose to the planned target volume. Beyond these advances, the continued evolution of more effective systems for delivery of radiation to the CNS may offer further benefit not only to adults but also to pediatric patients, a cohort of the population that may be more sensitive to the long-term effects of radiation. This article describes several novel irradiation techniques under investigation that hold promise in the pediatric population. These include newer approaches to intensity-modulated radiation therapy; stereotactic radiosurgery and radiation therapy; particle therapy, most notably proton therapy, which may be of particular benefit in enabling young patients to avoid radiation-related adverse effects; and radioimmunotherapy strategies that spare healthy tissue from radiotoxicity by delivering therapy directly to tumor tissue. Although emerging strategies for the delivery of radiation therapy hold promise for improved outcomes in pediatric patients, there must be rigorous long-term evaluation of consequences associated with the various techniques employed, to weigh risks, benefits, and impact on quality of life.
Introduction
This article is the second of a two-part series that examines concepts and approaches related to minimizing late treatment toxicities resulting from radiation therapy of pediatric central nervous system (CNS) tumors. Here, we discuss the specifics of refining current strategies for radiation delivery, as well as new and up-and-coming heavy particle techniques and radiotherapeutics.
Volumetric Modulated Arc Therapy (VMAT)
Modern radiation therapy involves the use of megavoltage photon energies to focus the dose on predetermined targets. Methods of radiation delivery include three-dimensional conformal radiation therapy (3D-CRT) using static fields, as well as newer methods of 3D-CRT intensity-modulated radiation therapy (IMRT), which uses computer-generated images to conform the shape of the radiation beam to the shape of the tumor as the beam exits the linear accelerator, in order to improve dose profiles and local tumor control. VMAT is a form of IMRT that involves rotation of the radiation gantry in an arc, with beam modulation. This modulated-arc approach provides multiple planes of treatment that enable normal tissue to be spared high doses of radiation but result in larger regions of low-dose scatter. The risks and benefits of conformal strategies vs irradiation techniques that generate a larger volume of low-dose scatter radiation continue to be debated.
Stereotactic Techniques
Stereotactic radiation delivery involves either a single dose, as in stereotactic radiosurgery (SRS); or a few large doses per fraction, as applied in stereotactic radiation therapy (SRT). These methods have been used both for dose escalation and to achieve ablative biologic effects. The approach is highly conformal and has been used with great success to treat a variety of malignant and benign diseases in adults. SRS/SRT may have a similar clinical application in the management of pediatric patients. The technique was first used employing Gamma Knife radiosurgery, requiring a headframe attached to the skull bone. However, with developments in stereotactic radiation therapy such as IMRT SRS/SRT, radiation can be delivered via a frameless technique. This approach has been used in the adult population. In 2014, Nanda et al reported their experience using a frameless stereotactic technique in the re-irradiation of recurrent pediatric CNS lesions. They found the technique was feasible in their 5 young patients, avoided trauma to the cranium, and decreased the amount of time that patients spent under general anesthesia.[1]
Charged-Particle Radiation Therapy
Standard radiation therapy for CNS diseases uses photon radiation, which transmits energy via electromagnetic waves. Charged-particle radiation therapy is an alternative method of radiation energy transfer that has been studied in recent decades, with two of the most promising approaches being proton and carbon ion radiation therapy. Particle therapy has found a unique niche in the pediatric population and is of particular importance in the treatment of CNS diseases, for which both the radiation dose to surrounding organs at risk and the integral dose are related to late toxicity and the risk of second malignancies.[2-6] There are 48 particle therapy centers in operation worldwide (as of 2014); proton therapy is used in more than 100,000 patients, and more than 13,000 patients are treated with carbon ion therapy.[7] While our understanding of the physics and radiobiology of protons is gradually improving, until recently the widespread incorporation of this technology has been limited by high associated costs and technical difficulties. Although the rationale for the use of charged-particle therapy is intriguing and logical, and there has been some pressure from the pediatric medical community to incorporate this technique into the management of children and adolescents with cancer, collection and analysis of data related to its use are still in their infancy.
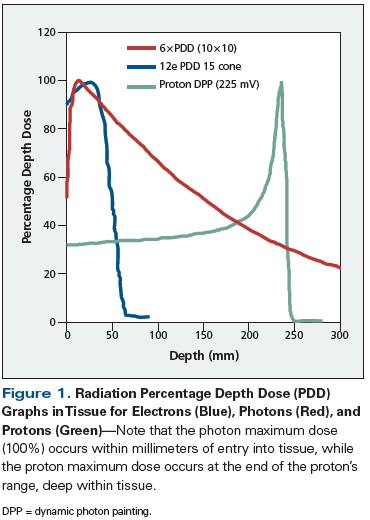
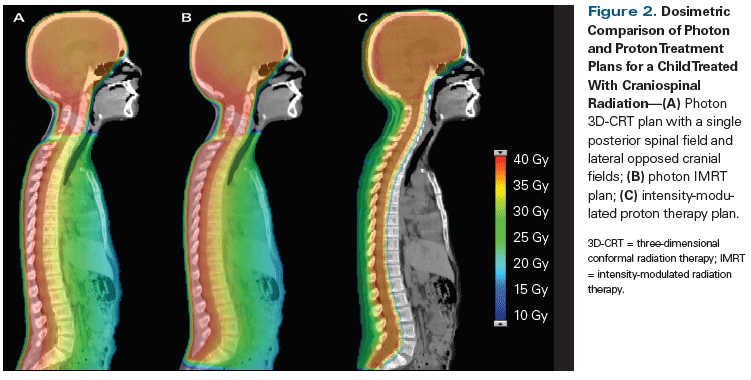
Charged particles produce an inverse dose distribution, in which the radiation dose builds slowly upon entering tissue. The majority of the energy is deposited at the end of the range of these particles, producing what is known as a Bragg peak (Figure 1). The peak can be modulated to allow for coverage of a treatment target, while minimizing dose to the tissue beyond and lateral to its range. This ability to control the shape and energy of the peak is in contrast to megavoltage photon therapy, which builds to maximum dose deposited just below the surface of tissue and slowly tapers off, resulting in a higher percent exit dose. This quality of conformability of dose distribution to critical targets is the rationale for implementing proton therapy in patients receiving craniospinal irradiation, in whom significant sparing of internal organs such as the lungs, heart, bowels, etc, can be achieved (Figure 2).
Modeling of proton therapy suggests a reduced volume of brain irradiated to low doses with this approach, resulting in a lower predicted risk of necrosis compared with VMAT.[6] MacDonald et al, in a study published in 2011, compared the whole-ventricle radiation plans of 3D treatment vs conformal protons, IMRT protons, and IMRT photons. They found that these techniques produced similar coverage of the targets, but that intensity-modulated proton therapy had superior dose homogeneity and sparing of critical structures.[8] Among the critical structures spared are the temporal lobes, damage to which has been associated with neurocognitive decline in the pediatric population.[9]
Carbon ions are similar to protons in their charged nature; however, they are heavy ions and have a higher relative biologic effectiveness than photons and protons. Carbon ion interaction with tissue causes fragmentation, resulting in the production of low-energy ions of low atomic number. The radiation dose distributions of carbon ion therapy, and the ability to achieve early local control, suggest the potential for clinical efficacy.[10]
If the ultimate goal of particle therapy is to reduce radiation toxicity to normal tissues, then naturally it is important to evaluate whether this is achieved. However, there is a notable paucity of high-level data regarding both the efficacy and toxicities of proton therapy and carbon ion therapy.[11] Current nonrandomized data suggest an acceptable and expected level of acute toxicity.[10,12] However, it is the late toxicities associated with treatment that have been identified as the most important consideration in radiation delivery to pediatric patients. Effects on neurocognitive function after proton therapy suggest that the patient’s age at irradiation, the radiation dose intensity, and the treatment volume affect the degree of cognitive decline that usually manifests 1 year to 2 years posttreatment.[13,14] In a prospective cohort study of 45 medulloblastoma patients treated with cerebrospinal irradiation and boost, with or without chemotherapy, overall the Full Scale Intelligence Quotient (FSIQ) score decreased by an average of 1.5 points per year for the median 7 years of follow-up, with the majority of the deficit being in processing speed and verbal comprehension. Endocrine effects were seen in 55% of patients, and ototoxicity was found in 16% of the patients at 5 years.[14]
In a recently published report, Pulsifer et al reviewed their cohort of 60 patients treated with proton therapy and found degrees of toxicity similar to those observed in published photon cohorts. At 2.5 years median follow-up, the authors found preserved cognitive function in their evaluation of the FSIQ score, except in the realm of mean processing speed, where the most significant change was seen in patients younger than 12 years of age.[13] Recent studies in the medical literature have also highlighted the need for proton-specific toxicity analysis, and potentially for proton-specific dose constraints. Reporting on their cohort of 313 patients who received proton therapy for brain or skull base tumors, Indelicato et al cited a 2.1% rate of grade 3 or higher brainstem toxicity.[15] In response to this need for prospective data, the Pediatric Proton Consortium Registry was established; the goal of the registry is to further our understanding of patterns of care, follow-up, and acute and late toxicities associated with the use of proton radiation therapy by medical institutions in the United States.[16]
KEY POINTS
- Novel radiation techniques and modalities aim to increase the therapeutic index of pediatric radiation.
- Advances in the delivery of photon therapy have allowed for conformal dose delivery to tumor, while minimizing dose to normal tissues.
- The use of charged or heavy particle radiation is currently an area of active research in pediatric radiation oncology and may afford unique benefits in preventing late complications in this population.
- Radioimmunotherapy is a promising modality of radiation delivery that allows for delivery directly to tumor tissues, and will require further investigation.
An additional consideration in the adoption of particle therapy is the significant infrastructure and cost requirements of this approach. A systematic review of the cost-effectiveness of proton therapy evaluated five pediatric trials; the authors found that, despite the higher initial infrastructure-related costs, there was a cost benefit to the use of proton therapy, primarily due to decreased rates of adverse events.[17] However, recent studies again highlight the current deficiency of high-level evidence for efficacy, noting that as radiation therapy technology evolves at a rapid pace, the cost-benefit analysis must be reweighed over time.[11,17]
Radioimmunotherapy (RIT)
RIT is a novel radiation technique recently applied to the treatment of pediatric patients with CNS malignancies. RIT delivers radioactive substances directly to tumor tissue by binding radiotracers to monoclonal antibodies directed against tumor antigens. Via this mechanism, monoclonal antibodies concentrate at tumor locations to deliver high doses of radiation to the target area of interest while sparing normal tissue.
RIT has been widely applied in the treatment of the most radiosensitive cancers, such as leukemia and lymphoma, but this technique has also shown promise in pediatric CNS tumors via intrathecal administration. Limitations of this therapy include its method of delivery and effects on metabolism. Using a two-compartment model of RIT with 131I-3F8 injected into the cerebrospinal fluid, He et al found that increasing the immunoreactivity of 131I-3F8 from 10% to 90% increased the therapeutic index by 7.4-fold, and delivered doses in the range of 100 Gy to tumor but less than 10 Gy to normal tissue.[18]
In early-phase trials, 131I-3F8 and 131I-8H9 have been used intrathecally as therapy for CNS metastases, and as treatment of leptomeningeal dissemination in patients with ependymoma, CNS primitive neuroectodermal tumor, medulloblastoma, neuroblastoma, rhabdoid tumors, and melanoma. In these trials, multiple clinical responses and extended progression-free survival durations were recorded, with acceptable levels of acute toxicity.[19,20] Promising applications of RIT in the management of CNS malignancies include the use of novel isotope-antibody conjugates, with studies now underway in both animal and human models.[21,22] Other novel techniques of RIT delivery, such as convection-enhanced RIT, directly implant the therapeutic agent into target tumor tissue (via minimally invasive surgery followed by insertion of a small infusion catheter) to further increase the drug concentration in tumor cells.
Conclusion
Future care of pediatric patients with CNS tumors holds the dual promise not only of treatment modalities that are increasingly being refined to achieve radiation dosing with a higher therapeutic index, but also the emergence of alternative techniques for radiation delivery that may further reduce risks of acute and long-term adverse effects. These benefits are of particular relevance to pediatric patients, given concerns about effects on growth and development, and the risk of second malignancies. It is hoped that rigorous patient evaluation and monitoring in clinical trials will clarify the true risks and benefits of the newest options for administering radiation therapy to pediatric patients with CNS tumors.
Acknowledgment: This research was supported in part by the intramural research program at the National Institutes of Health, National Cancer Institute, Radiation Oncology Branch, funded by grant ZID BC 010990.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Nanda R, Dhabbaan A, Janss A, et al. The feasibility of frameless stereotactic radiosurgery in the management of pediatric central nervous system tumors. J Neurooncol. 2014; 117:329-35.
2. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277-82.
3. Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705-17.
4. Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. 2016;31:237-54.
5. Inskip PD, Sigurdson AJ, Veiga L, et al. Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys. 2016;94:800-7.
6. Freund D, Zhang R, Sanders M, Newhauser W. Predictive risk of radiation induced cerebral necrosis in pediatric brain cancer patients after VMAT versus proton therapy. Cancers (Basel). 2015;7:617-30.
7. Jermann M. Particle therapy statistics in 2014. Int J Part Ther. 2015;2:50-4.
8. MacDonald SM, Trofimov A, Safai S, et al. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79:121-9.
9. Brodin NP, Munck af Rosenschold P, Blomstrand M, et al. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro Oncol. 2014;16:594-602.
10. Combs SE, Kessel K, Habermehl D, et al. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol. 2013;52:1504-9.
11. Leroy R, Benahmed N, Hulstaert F, et al. Proton therapy in children: a systematic review of clinical effectiveness in 15 pediatric cancers. Int J Radiat Oncol Biol Phys. 2016;95:267-78.
12. Rieber JG, Kessel KA, Witt O, et al. Treatment tolerance of particle therapy in pediatric patients. Acta Oncol. 2015;54:1049-55.
13. Pulsifer MB, Sethi RV, Kuhlthau KA, et al. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93:400-7.
14. Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17:287-98.
15. Indelicato DJ, Flampouri S, Rotondo RL, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014;53:1298-304.
16. Kasper HB, Raeke L, Indelicato DJ, et al. The Pediatric Proton Consortium Registry: a multi-institutional collaboration in U.S. proton centers. Int J Part Ther. 2014;1:323-33.
17. Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483-501.
18. He P, Kramer K, Smith-Jones P, et al. Two-compartment model of radioimmunotherapy delivered through cerebrospinal fluid. Eur J Nucl Med Mol Imaging. 2011;38:334-42.
19. Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409-18.
20. Kramer K, Pandit-Taskar N, Zanzonico P, et al. Low incidence of radionecrosis in children treated with conventional radiation therapy and intrathecal radioimmunotherapy. J Neurooncol. 2015;123:245-9.
21. Vera DR, Eigner S, Henke KE, et al. Preparation and preclinical evaluation of 177Lu-nimotuzumab targeting epidermal growth factor receptor overexpressing tumors. Nucl Med Biol. 2012;39:3-13.â©
22. Luther N, Zhou Z, Zanzonico P, et al. The potential of theragnostic 124I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma. Neuro Oncol. 2014;16:800-6.