(P089) A Phase III Randomized Trial of MRI-Mapped Dose-Escalated Salvage Radiotherapy Postprostatectomy: The Maps Trial-An Initial Dosimetric Assessment
Long-term salvage rates for men who undergo standard fraction radiation therapy (SFRT) due to biochemical failure after prostatectomy remain suboptimal. There is a poor understanding as to which patients are curable with standard salvage doses of radiation.
Table P089
Amber Orman, MD, Alan Pollack, MD, PhD, Radka Stoyanova, PhD, Kelin Wang, PhD, Adrian Ishkanian, MD, Matthew Abramowitz, MD; University of Miami Miller School of Medicine
Purpose and Objectives: Long-term salvage rates for men who undergo standard fraction radiation therapy (SFRT) due to biochemical failure after prostatectomy remain suboptimal. There is a poor understanding as to which patients are curable with standard salvage doses of radiation. Compared with definitive prostate treatment, dose to the prostate bed is limited by the absence of a clearly defined target and the need to treat a large area of potential contamination into which the bladder has been pulled. Multiparametric magnetic resonance imaging (MRI) has enabled us to identify small foci of residual or recurrent disease in approximately 40% of patients-foci of disease that are not identifiable on CT, ultrasound, or physical exam. As demonstrated in definitive prostate cancer irradiation, dose escalation to these foci may show a benefit. The MAPS trial is currently underway to evaluate whether a simultaneous incorporated hypofractionated boost (SIHB) to these areas compared with SFRT improves biochemical control. This trial evaluates 68 Gy in 34 fractions to the prostate bed, vs the same dose and volume with the addition of 2.25 Gy daily SIHB to the MRI-identified gross tumor volume (GTV) (biologically effective dose [BED] = 80 Gy, α/β = 1.5).
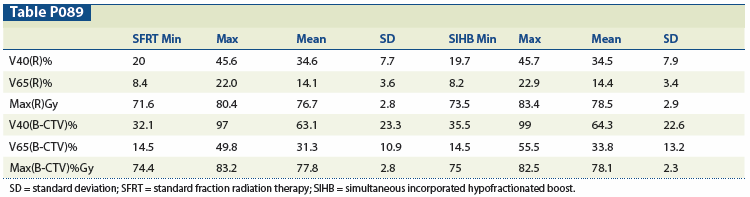
Materials and Methods: Two plans were generated for 14 patients treated per the MAPS protocol, one for each arm. 3D volumes of the MRI-identified GTV were generated in the Varian Eclipse treatment planning system (version 11.0). Intensity-modulated arc therapy was utilized for all plans. The trial stipulates that no more than 35% and 55% of the rectum (R) should receive ≥ 65 Gy and ≥ 40 Gy, respectively, and no more than 50% and 70% of the bladder minus the prostate bed clinical target volume (B-CTV) should receive ≥ 65 Gy and ≥ 40 Gy, respectively. Doses to targets and normal tissues were compared.
Results: Prostate bed CTV volumes ranged from 84.8 to 202.7 cc, with a mean (standard deviation [SD]) of 143.3 cc (39.2). GTV volumes ranged from 0.31 to 10.4 cc, with a mean (SD) of 2.34 cc (2.8). The table contains normal tissue criteria, which were achieved for all variables aside from bladder. Five plans had > 70% of the bladder receiving ≥ 40 Gy, and one plan had > 50% of the bladder receiving ≥ 65 Gy; these were considered secondary protocol variations and appeared to be due to suboptimal bladder filling. The PTV coverage ranged from 95.0% to 97.9%. The GTV coverage ranged from 95.0% to 100%. In terms of dosimetric constraints, as well as PTV coverage, there was no difference between the SIHB plans and SFRT plans per patient or overall.
Conclusions: This study demonstrates that even though most GTVs are located in close proximity to critical structures, the escalated dose scheme as described in the MAPS trial can be achieved without exceeding the toxicity tolerance specified for the rectum in all cases and the bladder in the majority, with expected variations due to unavoidably small bladders being entirely in the CTV. Treating MRI-identified prostate bed lesions to definitive doses using a SIHB is possible without increased dose to critical structures. Long-term follow-up will determine the biochemical outcome.
Targeted Therapy First Strategy Reduces Need for Chemotherapy in Newly Diagnosed LBCL
December 7th 2025Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone may allow 57% of patients with newly diagnosed LBCL to receive less than the standard number of chemotherapy cycles without compromising curative potential.