The activity and therapeutic licensing of poly(ADP-ribose) polymerase (PARP) inhibitors is the culmination of 50 years of research. However, the biology, mechanisms of action, adequate treatment combinations, and targeted populations for these agents need to be explored further. PARP activity is essential for the repair of single-strand DNA breaks via the base excision repair pathway. This pathway is the default repair pathway in cells with deficient high-fidelity double-strand break homologous recombination (HR) repair, such as occurs with loss of BRCA1 or BRCA2 function. Therefore, inhibition of PARP function results in cell death in HR-deficient tumors, and sensitizes tumor cells to cytotoxic agents that induce DNA damage. Applications of PARP inhibition are now being expanded beyond tumors with HR deficiency-to HR-competent tumors in which HR has been synthetically impaired through use of other agents given in combination with PARP inhibitors, or resulting from PARP inhibition in the setting of BRCA1 or BRCA2 loss.
Introduction
The discovery of the first poly(ADP-ribose) polymerase (PARP) was made over 50 years ago in Paul Mandel’s laboratory, with the observation of the synthesis of a new polyadenylic acid after nicotinamide mononucleotide had been added to rat liver extracts.[1] By 1980 it was known that this nuclear enzyme was activated by DNA damage and played an essential role in the repair of DNA.[2-4] There are now 17 known members of the PARP nuclear superfamily, with PARP1 and PARP2 as the two that are predominantly involved in DNA repair.[5]
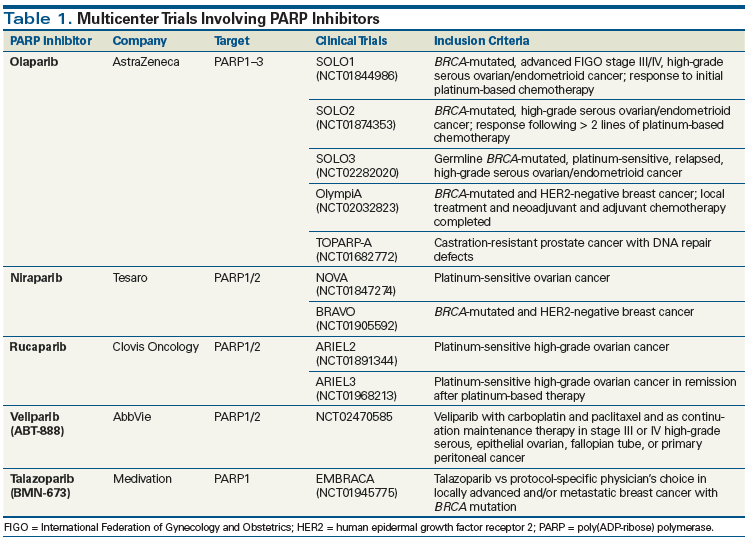
It was discovered that in cancer cells in which repair of DNA is already impaired, inhibition of PARP, by increasing genomic instability, can result in the death of the tumor cells.[6] The antitumor effects of PARP inhibition were first demonstrated in ovarian cancer cells.[3,4,7,8] Olaparib was the first PARP inhibitor to be approved by the US Food and Drug Administration (FDA); it was granted accelerated approval for use in BRCA1/2 mutation carriers in the fourth or greater treatment line, based on clinical data reporting antitumor activity that resulted in significant clinical benefit in high-grade serous ovarian cancer (HGSOC) with germline mutations in the BRCA1 or BRCA2 gene. Olaparib is approved in Europe for women with either germline or somatic deleterious mutations for maintenance of chemotherapy response to second- or later-line platinum-based therapy, after completion of the last platinum regimen. Olaparib has also been approved by Health Canada.
Ongoing phase II and III clinical trials are testing the efficacy of currently available PARP inhibitors-olaparib, niraparib, rucaparib, veliparib, and talazoparib-that have also shown antitumor efficacy and tolerability alone or when adding a synergistic effect in combination with other agents (Table 1). Rucaparib just received FDA approval in December 2016 for use in women with HGSOC in the third or greater line, especially patients with homologous recombination (HR) dysfunction as shown by an HR dysfunction companion diagnostic. Niraparib activity in maintenance of chemotherapy response in patients with recurrent ovarian cancer was reported in November 2016 and is awaiting review by the FDA and the European Medicines Agency (EMA).
DNA Repair and the Role of PARP
Two major forms of DNA damage can occur: single-strand breaks (SSBs) and double-strand breaks (DSBs). Thus, there are two repair pathway categories: SSB-targeted and DSB-targeted repair pathways. Base excision repair (BER) is one of several pathways involved in the repair of selected types of DNA SSBs. PARP1, via a process known as PARylation (poly[ADP-ribose] polymerization), plays an important role in the BER of DNA SSBs. In the nucleus, PARP1 senses SSB DNA injury and recruits DNA repair complexes to the site of SSBs (Figure 1).[9]
If PARylation is inhibited and BER is impaired, unrepaired or misrepaired SSBs accumulate, and at replication forks they degenerate to become DSBs. DSBs are repaired predominantly by the high-fidelity HR repair pathway that is active in the G2 phase of the cell cycle. The low-fidelity nonhomologous end-joining (NHEJ) program, which functions predominantly in the G1/S transition and S phase, is a poor-quality DSB repair backup; this form of repair religates DNA strands indiscriminately, introducing new DNA errors that cumulatively can result in cell death. (Figure 2).
BRCA1 and BRCA2 are tumor suppressor genes known for the association of their deleterious germline mutations with familial, high-penetrance breast and ovarian cancers.[10] Both BRCA1 and BRCA2 are involved in HR repair of DSBs and help maintain genomic stability. Cells with homozygous loss of normal BRCA1/2 function have defective HR and must rely more on the low-fidelity NHEJ program to repair DNA DSBs. Thus, cells with deleterious mutations in BRCA1 or BRCA2 are more dependent on BER and PARP1 for rescue and maintenance of genomic stability-and consequently more susceptible to impairment of the BER pathway. In such cells, PARP inhibition results in further genomic instability and cell death.[11]
In addition to tumors with deleterious germline BRCA1 (gBRCA1) or gBRCA2 mutations, other tumors in which HR is dysfunctional, such as those in which HR dysfunction results from genomic events besides germline mutations-eg, homozygous loss by somatic mutation, silencing by methylation, or loss of other HR proteins, such as RAD51c-are also more susceptible to PARP inhibition. In such settings, a program of clinical “synthetic lethality” is activated. Synthetic lethality exists when two conditions that independently would not cause cell death are applied in combination and together cause lethal injury.[12,13] Cancer cells already have impaired DNA repair function and a higher burden of genomic injury than normal cells; in tumors in which there has also been genomic loss of DNA repair function or other impairment of HR, synthetic lethality, as induced by PARP inhibition, may be used to target tumor tissue selectively, with decreased toxicity in normal cells that have intact repair pathways and thus are more able to survive an onslaught of DNA repair inhibitors.[14,15]
Development of PARP Inhibitors as Viable Anticancer Agents
The finding that PARP inhibitors were at least additive with platinums and other agents and resulted in clinical synthetic lethality when applied to HR-deficient cells led to the development of several NAD-mimetic PARP inhibitors. It is unclear which of these agents’ many mechanisms of action are primary to their clinical activity. PARP inhibitors block the PARylation that normally occurs in response to DNA damage. All PARP inhibitors have the capacity to trap the PARP enzyme on injured DNA, preventing binding of incoming repair proteins. Both the binding and dissociation affinities are critical, as the longer the PARP enzyme is trapped on DNA (a result of higher affinity), the greater the benefit observed in preclinical models. The other major role of PARP inhibition is regulation of DNA-dependent protein kinase, catalytic subunit (DNA-PKcs), the rate-limiting enzyme involved in NHEJ. PARP1 keeps DNA-PKcs in an inactive mode, inhibiting NHEJ activity. Upon loss of PARP1, DNA-PKcs is phosphorylated and activated, thereby promoting the dysfunctional and injurious DSB repair performed by the NHEJ program.[16]
Olaparib
Olaparib is an orally administered small molecule with demonstrated clinical benefit in HGSOC patients with deleterious gBRCA or somatic BRCA (sBRCA) mutations.[17] The FDA approval of olaparib was based on an open-label trial of 298 gBRCA patients, of whom 193 had recurrent or relapsed ovarian cancer and had received several prior lines of therapy. The tumor response rate (RR) was 26.2% (95% CI, 21.3%–31.6%) overall, with RRs of 31.1% in ovarian (95% CI, 24.6%–38.1%), 12.9% in breast (95% CI, 5.7%–23.9%), 21.7% in pancreatic (95% CI, 7.5%–43.7%), and 50.0% in prostate (95% CI, 15.7%–84.3%) cancers, respectively. Stable disease > 8 weeks was observed in 42% of patients (95% CI, 36.0%–47.4%), including in 40% of those with ovarian cancer (95% CI, 33.4%–47.7%), 47% of those with breast cancer (95% CI, 34.0%–59.9%), 35% of those with pancreatic cancer (95% CI, 16.4%–57.3%), and 25% of those with prostate cancer (95% CI, 3.2%–65.1%). The most common adverse events were fatigue, nausea, and vomiting. Grade > 3 adverse events were reported for 54% of patients; anemia was the most common (17%).[18] Fong et al reported the first evidence of olaparib clinical activity in gBRCA patients, with a 40% RR and 3 patients (6%) maintaining Response Evaluation Criteria in Solid Tumors (RECIST) disease stabilization for ≥ 4 months. The most common drug-related toxicities were the class-based toxicities of nausea (grade 1, 36%; grade 2/3, 36%), diarrhea (grade 1, 4%; grade 2/3, 2%), fatigue (grade 1, 8%; grade 2, 32%), and anemia (grade 2, 6%; grade 3, 8%).[19]
A randomized, double-blind, placebo-controlled, phase II study evaluated olaparib maintenance treatment in women with platinum-sensitive relapsed HGSOC who had received two or more platinum-based regimens. Patients with a complete response (CR) or partial response (PR) or stabilization of their disease on platinum-based therapy were randomized to placebo or olaparib 400 mg orally twice daily. The primary endpoint was progression-free survival (PFS); 265 patients underwent randomization. PFS was significantly longer with olaparib (median, 8.4 vs 4.8 months; hazard ratio, 0.35; 95% CI, 0.25–0.49; P < .001). Adverse events more commonly reported in the olaparib group vs in the placebo group were nausea (68% vs 35%), fatigue (49% vs 38%), vomiting (32% vs 14%), and anemia (17% vs 5%); the majority of adverse events were grade 1 or 2.[17] This demonstration of a benefit for olaparib as maintenance therapy was the basis for the EMA and Health Canada approvals.[17,20]
Additional trials are ongoing, examining olaparib in earlier settings for gBRCA women, and evaluating novel olaparib combinations. SOLO1 and SOLO2 (ClinicalTrials.gov identifiers: NCT01844986, NCT01874353) are double-blind, placebo-controlled, multinational, phase III studies in which patients are being randomized (2:1) to receive olaparib or placebo. Both of these trials include patients with gBRCA HGSOC. SOLO1 is testing olaparib as immediate front-line maintenance therapy for women who have achieved a PR or CR following platinum-based adjuvant chemotherapy. SOLO2 is following the original maintenance therapy design, examining the role of olaparib in maintenance of platinum-based therapy response in second- and later-line patients (see Table 1).
Olaparib also has been studied outside of ovarian cancer. A phase II proof-of-concept study resulted in antitumor activity in patients with recurrent, advanced gBRCA breast cancer, with an RR of 41% (ClinicalTrials.gov identifier: NCT00494234).[21] The ongoing phase III OlympiA trial (ClinicalTrials.gov identifier: NCT02032823) is testing olaparib as adjuvant monotherapy in patients with gBRCA triple-negative breast cancer (human epidermal growth factor receptor 2–negative/estrogen receptor–negative/progesterone receptor–negative breast cancer) who have completed local treatment and neoadjuvant or adjuvant chemotherapy. Patients are being randomized 1:1 to receive olaparib or placebo for a maximum of 12 months. The primary objective is invasive disease–free survival (DFS). Secondary objectives include overall survival (OS), distant DFS, incidence of new cancers, health-related quality of life, safety, and tolerability.
Other tumors with DNA repair defects may be susceptible to PARP inhibitors. Prostate cancer is a heterogeneous disease with an unknown prevalence of germline mutations in DNA repair genes. However, inherited mutations in DNA repair genes such as BRCA2 are associated with increased risk of metastatic prostate cancer (odds ratio, 2.2; 95% CI, 1.3–4.0; P = .002). A recent study assessed germline mutations in 20 DNA repair genes associated with autosomal dominant cancer predisposition syndromes in 692 patients with metastatic prostate cancer. Eighty-four germline, presumably deleterious, DNA repair gene mutations were identified in 82 men (11.8%); mutations were found in 16 genes, including BRCA2 (37 [5.3%]), ATM (11 [1.6%]), CHEK2 (10 [1.9% of 534 men with data]), BRCA1 (6 [0.9%]), RAD51D (3 [0.4%]), and PALB2 (3 [0.4%]). The frequency of mutations in DNA repair genes in men with metastatic prostate cancer significantly exceeded the prevalence of 4.6% among 499 men with localized prostate cancer (P < .001), as well as the prevalence of 2.7% in the Exome Aggregation Consortium, which includes 53,105 men without a known cancer diagnosis (P < .001). The relative risk of mutations in individual DNA repair genes among men with metastatic prostate cancer, as compared with men in the Exome Aggregation Consortium population, ranged from 18.6 (95% CI, 13.2–25.3; P < .001) for BRCA2, to 3.1 (95% CI, 1.5–5.6; P = .002) for CHEK2.[22] The 11.8% overall frequency of germline aberrations in genes responsible for maintaining DNA integrity in men with metastatic prostate cancer is substantially higher than the 1.2% to 1.8% incidence rate of BRCA2 mutations alone in men with localized prostate cancer[23,24] or the 7.3% incidence rate of mutations in 22 tumor suppressor genes in men with familial prostate cancer.[25]
It was hypothesized that olaparib would have activity in sporadic metastatic castration-resistant prostate cancer, and that this might be greater in men with potential underlying DNA repair defects (ClinicalTrials.gov identifier: NCT01682772). Patients enrolled had received prior chemotherapy and/or hormonal treatments; 16 of 49 evaluable patients responded (33%; 95% CI, 20%–48%), with 12 patients receiving study treatment for 6 months or longer. Next-generation sequencing identified germline homozygous deletions, deleterious mutations, or both, in DNA repair genes (including BRCA1/BRCA2, ATM, Fanconi anemia genes, and CHEK2) in 16 of 49 patients (33%). Of these 16 patients, 14 (88%) responded to olaparib, including all 7 with BRCA2 loss, and 4 of 5 with ATM aberrations. These results strongly argue that treatment with olaparib could benefit prostate cancer patients who were no longer responding to standard treatments and who carried deleterious defects in DNA repair genes.[26] Germline DNA repair gene mutations are not exclusive to early-onset prostate cancer and can be associated with clinically and histologically aggressive disease, while also potentially predicting for response to PARP inhibitors.
New findings also have indicated that loss of function of ATM can predispose to sensitivity to olaparib. Gastric cancer cell lines with low levels of ATM showed especial sensitivity to olaparib. A phase II study randomly assigned gastric cancer patients to olaparib 100 mg orally twice daily plus paclitaxel (80 mg/m2 IV weekly for 3 of 4 weeks [28-day cycle]) or placebo plus paclitaxel, followed by maintenance monotherapy with olaparib (200 mg orally twice daily) or placebo. In a cohort of 124 patients, the screening prevalence of low or undetectable ATM levels (ATMlow) was 14%. Olaparib/paclitaxel did not lead to a significant improvement in PFS in the whole population (median PFS, 3.91 vs 3.55 months; hazard ratio, 0.80) or in the ATMlow group (median PFS, 5.29 vs 3.68 months; hazard ratio, 0.74). However, olaparib/paclitaxel significantly improved OS in both the overall population (median OS, 13.1 vs 8.3 months; hazard ratio, 0.56; 80% CI, 0.41–0.75; P = .005) and the ATMlow population (median OS, not reached vs 8.2 months; hazard ratio, 0.35; 80% CI, 0.22–0.56; P = .002). Thus, olaparib/paclitaxel appears active in the treatment of metastatic gastric cancer, with a greater OS benefit in ATMlow patients.[27] A phase III trial in this setting is currently ongoing.
Niraparib
Niraparib is an oral inhibitor of PARP1 and PARP2. A phase I first-in-human study was expanded into separate cohorts enriched for HGSOC or castration-resistant prostate cancer. Antitumor activity was documented in both wild-type and gBRCA patients. Responses were achieved by 8 of 20 (40%) gBRCA ovarian cancer patients and 2 of 4 gBRCA breast cancer patients. Out of 22 women with BRCA wild-type HGSOC, 5 (23%) achieved a durable PR, and 9 of 21 (43%) patients with castration-resistant prostate cancer had stable disease, with a median duration of 254 days.[28]
Phase III studies with niraparib were initiated in 2013. The phase III, placebo-controlled, randomized (2:1) NOVA trial examined niraparib maintenance therapy (300 mg orally daily vs placebo) in patients with platinum-sensitive recurrent HGSOC (ClinicalTrials.gov identifier: NCT01847274); the primary endpoint was PFS. Eligible patients had histologically confirmed HGSOC (including fallopian tube and primary peritoneal cancers) or known gBRCA-associated ovarian cancer and had completed at least two previous courses of platinum-containing therapy. Two cohorts of patients were enrolled based on the presence or absence of the gBRCA mutation. Of the 533 enrolled patients, 203 had a gBRCA mutation and 350 did not. Patients in each cohort were randomized 2:1 to receive either niraparib (300 mg once daily) or placebo. Approximately 51% of patients achieved a CR, and the remainder achieved a PR; 39% of patients relapsed between 6 and 12 months after the penultimate platinum therapy, with 61% of relapses occurring at 12 months or later. In patients with a gBRCA mutation, median PFS was 21.0 months with niraparib vs 5.5 months with placebo (hazard ratio, 0.27; 95% CI, 0.173–0.410; P < .0001). Nonmutation carriers had a median PFS of 9.3 vs 3.9 months (hazard ratio, 0.45; 95% CI, 0.338–0.607; P < .0001). The median PFS was also significantly improved for patients with HR dysfunction but without a gBRCA mutation (12.9 vs 3.8 months; hazard ratio, 0.38; 95% CI, 0.254–0.586; P < .0001). The most common treatment-emergent severe adverse events were thrombocytopenia (28.3%), anemia (24.8%), and neutropenia (11.2%).[29]
BRAVO is an ongoing randomized, open-label, multicenter trial comparing niraparib vs physician’s choice in previously treated gBRCA breast cancer patients (ClinicalTrials.gov identifier: NCT01905592). Patients may have metastatic or locally advanced disease that is not amenable to resection or radiation therapy with curative intent, and may have received up to two prior cytotoxic regimens, with no prior cytotoxic regimens for advanced or metastatic disease (see Table 1).[30,31]
Rucaparib
Rucaparib was investigated initially in a phase I combination trial with temozolomide (TMZ) in adults with advanced solid tumors. Reduction of PARylation of DNA in peripheral blood mononuclear cells, as a measure of PARP inhibition, was seen at all tested doses in 33 patients. Initially, patients with solid tumors received escalating doses of rucaparib along with TMZ 100 mg/m2 daily × 5 every 28 days to establish the PARP inhibitory dose. Subsequently, the rucaparib dose was fixed at the PARP inhibitory dose and TMZ escalated to the maximum tolerated dose or 200 mg/m2 in patients with metastatic melanoma. The PARP inhibitory dose was 12 mg/m2, based on 74% to 97% inhibition of peripheral blood lymphocyte PARP activity. Recommended phase II doses were 12 mg/m2 for rucaparib and 200 mg/m2 for TMZ. Mean tumor PARP inhibition at 5 hours was 92% (range, 46% to 97%). All patients treated at the PARP inhibitory dose showed increases in DNA SSBs.[32]
Two rucaparib clinical trials are ongoing in ovarian cancer. ARIEL2 (ClinicalTrials.gov identifier: NCT01891344) is a phase II open-label trial of rucaparib, the aim of which is to develop the predictive potential of a molecular HR dysfunction signature in 180 women with relapsed platinum-sensitive measurable HGSOC. McNeisch et al have described a cutoff for application of an HR deficiency signature that appeared to predict rucaparib response.[33] This HR deficiency cutoff has been revised and is being applied prospectively for selection in the definitive phase III ARIEL3 trial (N = 540), an ongoing, randomized (2:1) placebo-controlled trial of maintenance therapy in patients with remitted platinum-sensitive recurrent HGSOC (see Table 1). The primary endpoint of ARIEL3 (ClinicalTrials.gov identifier: NCT01968213) is PFS by HR deficiency subgroup, as determined by the ARIEL2 optimized algorithm.
Veliparib (ABT-888)
Veliparib was shown in vivo to be a good inhibitor of PARP1 and PARP2. Like other PARP inhibitors, preclinical studies showed veliparib to be synergistic with therapies such as TMZ, platinums, cyclophosphamide, and radiation, all of which damage DNA.[34] Veliparib potentiated TMZ in a murine melanoma model. PARP inhibition dramatically increased the activity of TMZ in mice at veliparib doses as low as 3.1 mg/kg/day, with maximal activity achieved at 25 mg/kg/day. TMZ alone had minimal efficacy in an orthotopic gliosarcoma model, whereas TMZ with the addition of veliparib significantly slowed tumor progression. Veliparib also potentiated cisplatin, carboplatin, and cyclophosphamide in gBRCA breast xenograft models, causing regression of established tumors, whereas comparable doses of cytotoxic agents alone achieved only modest tumor inhibition. Finally, veliparib potentiated radiation (2 Gy/day × 10) in a colon carcinoma model. In each model, veliparib had no single-agent activity.
Subsequently, veliparib was combined with oral cyclophosphamide in an open-label, randomized, phase II study in patients with HGSOC and gBRCA ovarian cancers. Patients were randomized to receive cyclophosphamide (50 mg orally once daily), alone or with veliparib (60 mg orally once daily), in 21-day cycles. One CR was observed in each arm, with three PRs in the combination arm and six in the cyclophosphamide-alone arm, though without improvement in either the RR or the median PFS.[35,36] Genetic analyses were performed for 211 genes involved in DNA repair; none of the detected alterations were associated with treatment benefit. A phase III randomized trial of adjuvant chemotherapy of carboplatin paclitaxel chemotherapy with veliparib or placebo (ClinicalTrials.gov identifier: NCT02470585) in patients with newly diagnosed ovarian cancer is underway.
Talazoparib (BMN-673)
Talazoparib is 20-fold to more than 200-fold more potent on a molar level in vitro than other members of the NAD-mimetic class of PARP1/2 inhibitors. It is a strong PARP trapper, which may also account for its increased preclinical activity and its clinical toxicity at relatively equipotent doses. Oral administration of talazoparib elicited remarkable antitumor activity in a xenografted PARP inhibitor–resistant gBRCA breast tumor cell line (CAL-51). Synergistic or additive antitumor effects in colon cancer cell lines were also found when talazoparib was combined with TMZ, SN38 (an active metabolite of irinotecan), or platinum drugs.[37] In vivo studies reported that talazoparib induced statistically significant differences in event-free survival in 17/43 (39.5%) xenograft models from the National Cancer Institute’s Pediatric Preclinical Testing Consortium. Three objective responses were observed: a CR in a medulloblastoma line (BT-45), a maintained CR in a Wilms tumor line (KT-10), and a maintained CR in an ependymoma line (BT-41). Talazoparib maintained its high level of activity against KT-10 with a threefold reduction in dose. KT-10 possesses a truncating mutation in PALB2, analogous to PALB2 mutations associated with hereditary breast and ovarian cancer that abrogate HR repair.[38]
The ongoing phase III EMBRACA international trial is comparing the safety and efficacy of talazoparib vs physician’s choice (capecitabine, eribulin, gemcitabine, or vinorelbine) in patients who have locally advanced and/or metastatic gBRCA breast cancer (ClinicalTrials.gov identifier: NCT01945775). The primary objective is PFS and secondary objectives include RR, OS, and safety (see Table 1).
Future Directions for Single-Agent PARP Inhibitors
PARP inhibitors, as single agents, may have a more focused application in the treatment of cancers with HR deficiency, either resulting from gBRCA mutations, and in other cases not directly due to BRCA mutations. Up to half of HGSOCs evaluated in the Cancer Genome Atlas molecular analysis have been postulated to be HR deficient. This “HR-deficient” designation includes tumors with sBRCA mutations (6% to 8%) and epigenetic silencing in genes not associated with gBRCA but essential to HR repair, such as ATM, CHEK2, RAD51, and MRE11A. Similarly, using a functional assay of HR repair, and sensitivity to PARP inhibitors as indicators of HR-deficient status, 55% of unselected HGSOCs were found to have HR deficiency.[39] Potential selection HR deficiency signatures are under development in the NOVA and ARIEL2/3 trials. The tripartite HR deficiency signature codeveloped by academia and Myriad Genetics also may be promising in cancers outside of HGSOC.
Combination Approaches
A therapeutic approach to induction of an HR-deficient phenotype in otherwise HR repair–competent cancers is a way to expand the therapeutic opportunities for PARP inhibitors and future DNA repair inhibitors. It has been hypothesized that combining PARP inhibitors with inhibitors of other signaling pathways may create a clinical synthetic lethality, and obviate dependency upon gBRCA. There are many ways to create clinical synthetic lethality, including, but not limited to, induction of mutations by therapeutic or ambient microenvironmental changes, generation of oxygen radicals, and alteration of the tumor microenvironment through hypoxia.[40]
KEY POINTS
- PARP inhibitors have been shown to be associated with a progression-free survival benefit in patients with germline BRCA (gBRCA)-mutated, homologous recombination (HR)-deficient ovarian cancer, leading to their approval with a significant impact on improving outcomes.
- Activity in wild-type and gBRCA1/2 and HR-deficient tumors is being explored in both ovarian cancer and other tumor types.
- New combinations with other targeted therapies will be of great interest during the next few years.
Preclinical studies demonstrated the additive effect of combining antiangiogenesis with PARP inhibition, with hypoxia leading to downregulation of HR proteins and enhanced PARP inhibitor sensitivity. Repression of BRCA1 expression by hypoxia represents a mechanism of functional BRCA1 inactivation in the absence of genetic mutation. Hypoxia, a common feature of solid tumors, may cause local loss of BRCA1 in many sporadic cancers, leading to possible treatment benefit. It was demonstrated functionally that hypoxia is associated with impaired HR, whereas the NHEJ repair pathway is unaffected by hypoxic conditions.[41-43]
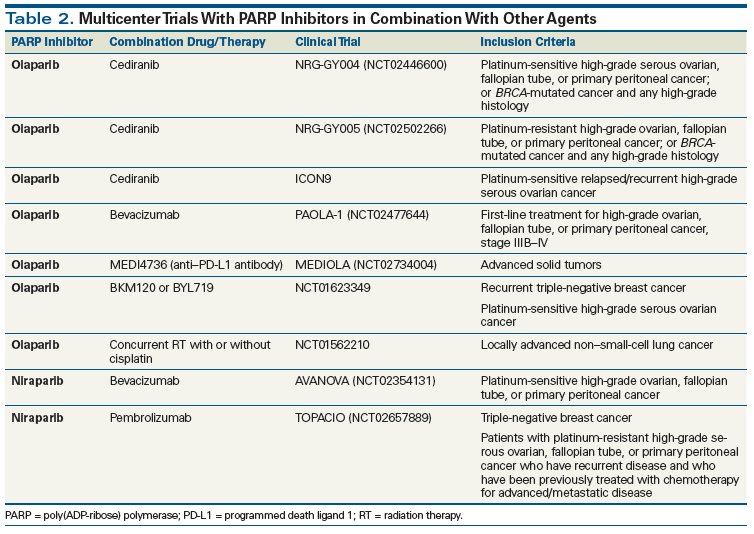
A phase I trial combined cediranib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, with olaparib, yielding both safety and an RR of 44% in recurrent ovarian cancer.[44] This led to a randomized phase II study comparing olaparib with the combination. The median PFS for women who received cediranib plus olaparib was 17.7 months (95% CI, 14.7 months–not reached), compared with 9.0 months (95% CI, 5.7–16.5 months) for those treated with olaparib monotherapy (hazard ratio, 0.42; 95% CI, 0.23–0.76; P = .005). A post-hoc unplanned subset analysis examined differences in behavior as a function of gBRCA status. The distribution of gBRCA was balanced between arms and approximated half the cohort. Women who were gBRCA-unknown or gBRCA-negative had the greatest benefit from cediranib plus olaparib as compared with olaparib alone, with a nearly threefold higher PFS (16.5 vs 5.7 months; hazard ratio, 0.32; 95% CI, 0.14–0.74; P = .008); a smaller benefit was observed for cediranib plus olaparib in gBRCA women (PFS, 19.4 vs 16.5 months; hazard ratio, 0.55; 95% CI, 0.24–1.27; P = .16).
Women with platinum-resistant disease are less commonly gBRCA. The finding that cediranib plus olaparib improved PFS in women with recurrent platinum-sensitive gBRCA-negative HGSOC led to the hypothesis that this combination may benefit platinum-resistant disease.[45] Two randomized phase III registration studies have begun combining olaparib with cediranib in women with platinum-sensitive HGSOC (ClinicalTrials.gov identifier: NCT02446600), and in women with platinum-resistant ovarian cancer (ClinicalTrials.gov identifier: NCT02502266). Studies have been initiated to capitalize on this potential clinical synthetic lethality, with other antiangiogenic agents, other PARP inhibitors, and in other cancers (Table 2).
Expanding the role of PARP inhibitors into other cancer types by leveraging combination activity is now being broadened. TMZ is the mainstay of treatment in neuroendocrine tumors (NETs).[46] These tumors have no known mutations in HR DNA repair genes. However, NETs have been shown to have altered expression of O6-methylguanine-DNA methyltransferase (MGMT) and (in some cases) of DNA mismatch repair genes. There are preclinical PARP inhibitor and TMZ synergy data and preliminary findings that suggest that MGMT-expressing cancers may be more susceptible to this combination.[47] PARP inhibitors used at low or submicromolar concentrations potentiated TMZ cytotoxicity in a variety of cell lines. Tentori et al reported that the use of the PARP inhibitor GPI 15427 together with TMZ was more active than TMZ alone in syngeneic murine melanoma or lymphoma models and in an orthotopic glioblastoma multiforme xenograft. In all models, administration of GPI 15427 before TMZ significantly increased the lifespan of tumor-bearing mice compared with untreated controls or monotherapy-treated groups.[48,49]
In the phase I trial of rucaparib with TMZ described previously, all patients treated at the PARP inhibitory dose of 12 mg/m2 showed increases in DNA SSBs in peripheral blood mononuclear cells and tumor biopsy posttreatment. Encouraging evidence of activity was seen.[32] A phase II study tested the combination in patients with advanced metastatic melanoma-intravenous rucaparib, 12 mg/m2, and oral TMZ, 200 mg/m2, on days 1–5 every 28 days. The RR was 17.4%, median time to progression was 3.5 months, median OS was 9.9 months, and 36% of patients were progression-free at 6 months. This study demonstrated the safety of TMZ combined with a PARP inhibitor.[50] These safety data, coupled with the defined dysfunction in DNA stability in NETs, show that use of PARP inhibitors with TMZ is an appealing option for this hard-to-treat rare tumor.
Conclusion
PARP inhibitors are an active, novel, and exciting class of anticancer agents. They have shown clear patient benefit in gBRCA, HR-deficient, and other ovarian cancers, leading to approval and a significant impact on ovarian cancer outcomes. Clinical trials are ongoing to advance our understanding of how and when these DNA repair inhibitors can be best used-in first line, as maintenance therapy, and/or in recurrent disease-as well as how best to combine them, and with which other agents, in order to sensitize ovarian cancers and other tumors to PARP inhibitors’ DNA repair inhibitory effects. Both preclinical and clinical development of novel combinations will expand our knowledge of the role of DNA repair pathways, and our ability to further damage DNA, resulting in fatal genomic instability. It is hoped that this knowledge will lead to new PARP inhibitor combinations and treatments for new target diseases, and will extend the benefit of this exciting new therapeutic option to a broader array of cancer patients.
Financial Disclosure:This work was funded by the Intramural Program of the Center for Cancer Research (JDR) and the Cancer Therapy Evaluation Program (ECK), National Cancer Institute, National Institutes of Health. The authors have no disclosures.
References:
1. Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11:39-43.
2. Benjamin RC, Gill DM. ADP-ribosylation in mammalian cell ghosts. Dependence of poly(ADP-ribose) synthesis on strand breakage in DNA. J Biol Chem. 1980;255:10493-501.
3. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-7.
4. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-21.
5. Rouleau M, Patel A, Hendzel MJ, et al. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293-301.
6. Rouleau M, Patel A, Hendzel MJ, et al. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293-301.
7. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-30.
8. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643-6.
9. Morgan MA, Parsels LA, Maybaum J, Lawrence TS. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov. 2014;4:280-91.
10. Clamp A, Jayson G. PARP inhibitors in BRCA mutation-associated ovarian cancer. Lancet Oncol. 2015;16:10-2.
11. Drew Y. The development of PARP inhibitors in ovarian cancer: from bench to bedside. Br J Cancer. 2015;113(suppl 1):S3-S9.
12. Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689-98.
13. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371:1725-35.
14. Bryant H E, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-7.
15. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-21.
16. Zha S, Jiang W, Fujiwara Y, et al. Ataxia telangiectasia-mutated protein and DNA-dependent protein kinase have complementary V(D)J recombination functions. Proc Natl Acad Sci USA. 2011;108:2028-33.
17. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382-92.
18. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-50.
19. Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval.J Clin Oncol. 2010;28:2512-9.
20. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852-61.
21. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235-44.
22. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443-53.
23. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115-21.
24. Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230-4.
25. Leongamornlert D, Saunders E, Dadaev T, et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br J Cancer. 2014;110:1663-72.
26. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-708.
27. Bang YJ, Im SA, Lee KW, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol. 2015;33:3858-65.
28. Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882-92.
29. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-64.
30. Jones P, Wilcoxen K, Rowley M, Toniatti C. Niraparib: a poly(ADP-ribose) polymerase (PARP) inhibitor for the treatment of tumors with defective homologous recombination. J Med Chem. 2015;58:3302-14.
31. Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882-92.
32. Plummer R, Jones C, Middleton M, et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14:7917-23.
33. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
34. Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728-37.
35. Kummar S, Oza AM, Fleming GF, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21:1574-82.
36. Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation-an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137:386-91.
37. Shen Y, Rehman FL, Feng Y, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003-15.
38. Smith MA, Hampton OA, Reynolds CP, et al. Initial testing (stage 1) of the PARP inhibitor BMN 673 by the pediatric preclinical testing program: PALB2 mutation predicts exceptional in vivo response to BMN 673. Pediatr Blood Cancer. 2015;62:91-8.
39. Mukhopadhyay A, Elattar A, Cerbinskaite A, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344-51.
40. Ivy SP, Liu JF, Lee JM, et al. Cediranib, a pan-VEGFR inhibitor, and olaparib, a PARP inhibitor, in combination therapy for high grade serous ovarian cancer. Expert Opin Investig Drugs. 2016;25:597-611.
41. Bindra RS, Gibson SL, Meng A, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597-604.
42. Gibson SL, Bindra RS, Glazer PM. Hypoxia-induced phosphorylation of Chk2 in an ataxia telangiectasia mutated-dependent manner. Cancer Res. 2005;65:10734-41.
43. Chan N, Pires IM, Bencokova Z, et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. 2010;70:8045-54.
44. Liu JF, Tolaney SM, Birrer M, et al. A phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013;49:2972-8.
45. Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207-14.
46. Liu IH, Ford JM, Kunz PL. DNA-repair defects in pancreatic neuroendocrine tumors and potential clinical applications. Cancer Treat Rev. 2016;44:1-9.
47. Miknyoczki SJ, Jones-Bolin S, Pritchard S, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol Cancer Ther. 2003;2:371-82.
48. Tentori L, Leonetti C, Scarsella M, et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003;9:5370-9.
49. Tentori L, Portarena I, Barbarino M, et al. Inhibition of telomerase increases resistance of melanoma cells to temozolomide, but not to temozolomide combined with poly(ADP-ribose) polymerase inhibitor. Mol Pharmacol. 2003;63:192-202.
50. Plummer R, Lorigan P, Steven N, et al. A phase II study of the potent PARP inhibitor, rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother Pharmacol. 2013;71:1191-9.