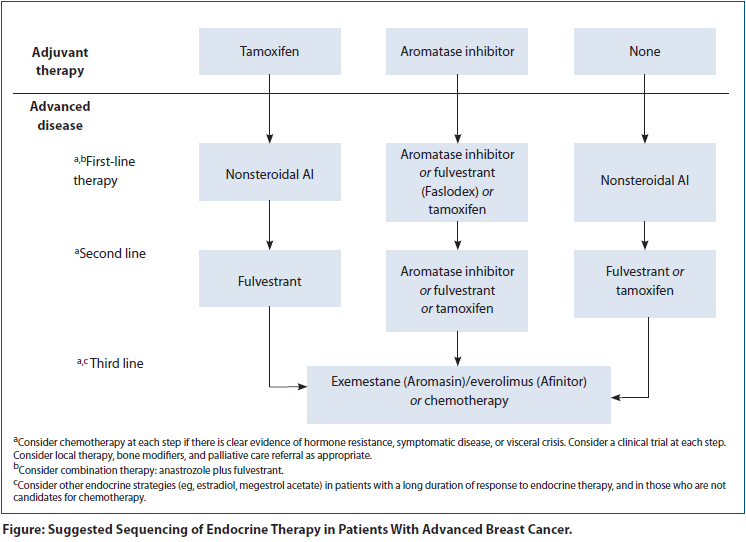
Advanced hormone receptor (HR)–positive breast cancer is characterized by expression of the estrogen receptor (ER) and/or progesterone receptor (PR), and typically has a more favorable prognosis than other breast cancer subtypes. Several factors predict survival for this patient subgroup, including the interval between early breast cancer diagnosis and disease relapse, the number and location of metastatic sites, and performance status. Evaluation of the patient with newly diagnosed metastatic disease involves tumor staging, biopsy of a metastatic site when feasible, and accurate (re)assessment of the ER, PR, and human epidermal growth factor receptor 2 (HER2) status.[1] The goals of managing this disease include prolongation of progression-free survival (PFS) and overall survival (OS), amelioration of symptoms, and improvement in quality of life. Postmenopausal women with HR-positive breast cancer may live with their disease for several years. A treatment paradigm that maximizes efficacy and minimizes toxicity is therefore of paramount importance.
Endocrine Therapy
Endocrine therapy is the most important component of the treatment paradigm for the majority of women with HR-positive advanced breast cancer. It is favored as initial therapy over alternative approaches such as chemotherapy, owing to its clinical activity and favorable safety profile. This approach is most appropriate when there is a slow progression and low burden of metastatic disease (including bone-only), and no impending visceral crisis. A meta-analysis of clinical trials comparing chemotherapy vs endocrine therapy indicated increased response rates with use of chemotherapy but no difference in OS.[2] The standard of care thus typically involves sequencing of endocrine agents until intolerance, development of resistance, and/or visceral crises necessitate a transition to chemotherapy (see Figure).
The ER has been the most important biomarker in breast cancer for more than 30 years, largely because of its role in predicting benefit from endocrine therapy. Several therapeutic options exist for blocking estrogen signaling. Tamoxifen is a selective estrogen receptor modulator (SERM) that binds to the ER and selectively inhibits transcriptional activity in breast cancer cells (estrogen antagonist), while functioning to activate the receptor in other tissues, including bone and endometrium (estrogen agonist). Common side effects of tamoxifen include hot flashes, vaginal discharge or dryness, and sexual dysfunction. In rare cases, tamoxifen can cause thromboembolic disease, stroke, endometrial cancer, or cataracts.[3] The aromatase inhibitors (AIs) function in a different manner by inhibiting the aromatase (CYP19A1) enzyme, which catalyzes conversion of androgens to aromatic estrogens in the adrenal gland and adipose tissue, resulting in profound estrogen deprivation in postmenopausal women. Anastrozole (Arimidex) and letrozole (Femara) are nonsteroidal AIs that bind reversibly to aromatase, while exemestane (Aromasin) is a steroidal AI that functions through irreversible binding and inactivation of the enzyme. The side-effect profile of the AIs differs from that of tamoxifen and includes hot flashes, vaginal dryness, musculoskeletal effects, reduction in bone mineral density, and osteoporosis, as well as potential for increased cardiovascular risk and hypercholesterolemia.[4] Fulvestrant (Faslodex) is an ER antagonist or selective ER down-regulator. Side effects include injection site pain, nausea, constipation, hot flashes, and bone and joint pain.[5] It is administered as an intramuscular injection with a recommended schedule of a 500-mg loading dose on days 1 and 14, and then monthly, based on a statistically significant increase in PFS observed in a phase III trial comparing the high dose to the originally approved dose of 250 mg monthly.[6]
Choice of Endocrine Therapy
The decision to choose one agent over another depends on a number of factors, including comorbidities (eg, history of thromboembolic disease, cardiovascular disease, osteopenia/osteoporosis), prior history of endocrine therapy use, prior tolerance to specific agents, or patient and/or physician preference.
For patients with HR-positive, HER2-negative metastatic disease, the response rate to first-line endocrine therapy with anti-estrogens such as tamoxifen or AIs ranges from 21% to 33%.[7-9] AIs generally represent the agent of initial choice in treating postmenopausal patients with advanced breast cancer. A meta-analysis of randomized controlled trials in patients with advanced breast cancer has demonstrated a survival advantage in women receiving AIs compared with alternative endocrine treatments in the first-line setting.[10] It remains unclear if one of the third-generation AIs is superior to the others, although the clinical data are limited. In a subsequent review comparing the effects of any AI vs another endocrine therapy, no endocrine therapy, or another AI in advanced breast cancer, Cochrane investigators observed a survival benefit of 10% with the use of AIs compared with other approaches for treatment of advanced breast cancer. Importantly, no significant differences in survival were observed between the different third-generation AIs.[11]
An increasing body of evidence also supports use of fulvestrant in this population of patients. Recent follow-up results from a phase II randomized, open-label study (n = 205) comparing fulvestrant at 500 mg with anastrozole at 1 mg as first-line endocrine therapy for postmenopausal women with HR-positive advanced breast cancer (FIRST trial; Fulvestrant fIRst-line Study comparing endocrine Treatments) were presented. Median time-to-progression (TTP) was 23.4 months for fulvestrant vs 13.1 months for anastrozole, consistent with a 34% reduction in risk of progression (hazard ratio [HR] = 0.66; 95% confidence interval [CI], 0.47–0.92; P = .01). OS data are not available at this time.[12] Ongoing investigations aim to further delineate the optimal use of fulvestrant.
Ongoing studies will also help delineate the optimal choice of second-line agents and beyond, and the optimal sequencing of endocrine therapies for this patient population. EFECT (The Evaluation of Faslodex vs Exemestane Clinical Trial) (n = 693) was a randomized, double-blind, placebo-controlled, multicenter phase III study of fulvestrant vs exemestane in postmenopausal women with HR-positive advanced breast cancer who had experienced disease progression or recurrence after treatment with a nonsteroidal AI. Both agents were well-tolerated and equally active in both groups, with a median TTP of 3.7 months, overall response rate (ORR) of approximately 7%, and clinical benefit rate of approximately 32%.[9] It must be noted that the fulvestrant dose used in this study was 250 mg, and not the currently recommended 500-mg dose. If a decision is made to administer an AI in the second-line setting, the efficacy of third-generation AIs appears comparable. When letrozole was compared with anastrozole as second-line endocrine therapy, the ORR was significantly higher with letrozole (19% vs 12%), but there was no significant difference in TTP (the primary endpoint) or in OS.[13] Exemestane and anastrozole are also associated with similar efficacy (in terms of ORR and OS) when administered to postmenopausal patients with visceral metastases.[14] Consideration might be given, however, to prescribing nonsteroidal AIs initially to facilitate subsequent use of the exemestane/everolimus (Afinitor) approach described later in this review. Other less-utilized endocrine manipulations include megestrol acetate (Megace) and low-dose estradiol. A study of low-dose estradiol as second- or third-line therapy has documented stable disease, but no objective responses.[15]
Combining Endocrine Therapies
Other studies have evaluated combination strategies of endocrine therapies compared with single agents. In the Southwest Oncology Group (SWOG) S0226 trial, postmenopausal women with newly diagnosed HR-positive advanced breast cancer were randomly assigned to either anastrozole or to the combination of anastrozole and fulvestrant (n = 707).[16] Fulvestrant was administered as a loading dose of 500 mg on day 1, followed by 250 mg on days 14 and 29, followed by 250-mg maintenance monthly thereafter. Overall, median PFS was 13.5 months for anastrozole and 15.0 months for the combination arm. The combination arm was also associated with improved median OS (41.3 months and 47.7 months, respectively). Adverse events did not differ significantly between the groups. The authors concluded that the combination of anastrozole and fulvestrant should be considered a new standard in the first-line treatment of metastatic HR-positive breast cancer in postmenopausal women. Again, it must be noted that the dose of fulvestrant used was lower than that currently recommended. In addition, a prior study with this combination of endocrine therapy in a less treatment-naive patient population did not reveal a difference in PFS between the arms.[17] Nonetheless, this combination strategy may be considered for patients with newly diagnosed HR-positive advanced breast cancer. Confirmation of results of the SWOG trial, however, are required before this treatment approach becomes widespread.
Novel Combination Strategies
Although pharmacologic therapies that reduce or block estrogen signaling are effective in the treatment of HR-positive breast cancer, de novo resistance is present in a large proportion of tumors. Virtually all tumors will develop acquired resistance, and several mechanisms have been proposed.[18] Aberrant signaling via the PI3K/Akt/mTOR intracellular signaling pathway has been associated with resistance to endocrine therapies; recent investigations have assessed endocrine therapy alone vs strategies combining endocrine therapies with novel agents that target this pathway.
The BOLERO-2 (Breast Cancer Trials of Oral Everolimus) investigators randomized postmenopausal women with HR-positive refractory metastatic breast cancer (with recurrence or progression following prior therapy with a nonsteroidal AI) to exemestane in combination with everolimus (Afinitor; a mammalian target of rapamycin [mTOR] inhibitor) or placebo.[19] Women could have received any prior number of endocrine treatments and a single prior chemotherapy. The trial met its primary endpoint of improvement in PFS by local review with the combination therapy (7.8 months) compared with the placebo arm (3.2 months). These results were confirmed by an independent, blinded radiologic assessment (PFS of 10.6 months vs 4.1 months, respectively). OS data are immature at this time. The most common grade 3/4 adverse reactions were stomatitis, infections, hyperglycemia, fatigue, dyspnea, pneumonitis, and diarrhea, all of which occurred more frequently in the combination arm. Adverse reactions resulting in permanent discontinuation were more frequent in the everolimus arm, as were dose interruptions or reductions. Based on the observed PFS benefit, this regimen has been approved for use by the US Food and Drug Administration for patients with metastatic breast cancer whose disease has progressed on a non-steroidal AI. Everolimus is the first agent to be approved for use in addition to endocrine therapy in patients with HER2-negative disease and is under investigation in earlier stages of the disease. When recommending this treatment approach for a patient, careful consideration should be given to the associated toxicities, and increased supportive care may be required. We consider this combination in patients who fit the eligibility criteria for the BOLERO-2 trial, usually as third-line therapy or beyond, or as a transition regimen prior to chemotherapy. A number of trials currently ongoing or in development are combining endocrine therapy with other inhibitors of the PI3K pathway. These include the phase III BELLE-2 trial, which randomizes postmenopausal patients with HR-positive, HER2-negative advanced breast cancer (refractory to AI) to a PI3K inhibitor/placebo with fulvestrant.[20] The studies will also investigate biomarkers predictive of benefit to the therapies, such as activation status of the PI3K pathway.
Exciting results were presented recently from the phase II TRIO-18 trial (n = 165), in which the combination of palbociclib (PD0332991), a cyclin-dependent kinase (CDK) 4/6 inhibitor, and letrozole was compared with letrozole alone in HR-positive advanced breast cancer (in the first-line setting).[21] This agent prevents cellular DNA synthesis by blocking cell cycle progression, and it was predicted in preclinical models to be of benefit in luminal breast cancer subtypes.[22] A significant improvement in PFS was observed for the combination arm compared with letrozole alone (median PFS, 26.1 months and 7.5 months, respectively). The most commonly reported treatment-related adverse events in the combination arm were neutropenia, leukopenia, anemia, and fatigue. A phase III trial is underway to attempt to confirm these results and identify predictive biomarkers of response to this therapy.[23]
Another potential mechanism of resistance to endocrine therapy includes epigenetic modifications, which might be modulated with the use of agents such as histone deacetylase (HDAC) inhibitors or demethylating agents. Epigenetics is a term that refers to changes in gene expression secondary to histone hypoacetylation and abnormal DNA methylation in the promoter region of important genes. Because of the frequency of detection of epigenetic alterations in breast cancers, agents that target these changes are of great interest. The ENCORE (ENtinostat Combinations Overcoming REsistance) 301 randomized phase II study evaluated the role of entinostat (MS-275, SNDX-275), a HDAC inhibitor, in combination with exemestane in the advanced breast cancer setting.[24] Postmenopausal women who had received ≤ 1 prior chemotherapy and had progressed on a non-steroidal AI were randomized to exemestane plus entinostat/placebo. A significant improvement in PFS was noted in the entinostat arm compared with placebo (median 4.3 months vs 2.3 months, respectively). Median OS, a secondary endpoint, was also significantly longer in the entinostat vs the placebo arm (28.1 months vs 19.8 months, respectively). The most common grade 3/4 toxicities were fatigue and neutropenia. Interestingly, in a subset analysis examining protein acetylation in entinostat-treated patients (n = 27), the median PFS increased to 8.5 months in those exhibiting protein lysine hyperacetylation vs 2.7 months for low acetylators, and this was apparent after just 2 weeks of therapy. A phase III trial is in development that will simultaneously evaluate the addition of entinostat to exemestane in patients who have and have not experienced disease progression on a nonsteroidal AI.
Other Therapeutic Strategies
HR-Positive Advanced Breast Cancer
- The goals of care for patients with hormone receptor (HR)-positive advanced breast cancer include prolongation of disease-free and overall survival, amelioration of symptoms, and improvement in quality of life.
- Use of endocrine therapy as an initial strategy, when clinically appropriate, is preferable, owing to both the expected clinical activity and favorable safety profile.
- Therapeutic strategies combining endocrine therapies with novel agents targeting aberrant molecular pathways, to overcome both de novo and acquired resistance to endocrine therapies, are promising and aim to improve outcomes for patients.
- Chemotherapy is appropriate for patients with HR-positive advanced breast cancer and an adequate performance status when there is evidence of endocrine resistance or intolerance to available endocrinebased treatment approaches, symptomatic disease, or visceral crisis.
Chemotherapy is recommended to patients with HR-positive advanced breast cancer and an adequate performance status, when there is evidence of disease progression or intolerance to available endocrine-based treatment approaches, symptomatic disease, or visceral crisis. We generally recommend sequential single-agent chemotherapy, except when a rapid response is required, such as in patients with visceral crisis. The choice of chemotherapy can include a taxane, an anthracycline, capecitabine (Xeloda), and eribulin (Halaven) amongst many options; it may be driven by physician or patient preference after education regarding potential benefits and side effects.
For patients with HR-positive, HER2-positive disease, consideration can be made for adding an anti-HER2 agent to endocrine therapy, including the combination of anastrozole plus trastuzumab (TAnDEM [Trastuzumab in Dual HER2 ER-positive Metastatic breast] trial)[25] and letrozole plus lapatinib (Tykerb).[26] For patients with slow-growing disease and an absence of symptoms or visceral crisis, serial combinations of anti-HER2 therapy plus endocrine therapy can be employed prior to the initiation of a chemotherapy-based approach. In the case of minimal disease burden, we also consider an endocrine therapy–alone approach initially, and add the anti-HER2 therapy upon progression.
Supportive care measures such as the administration of bone-modifiers (eg, bisphosphonates, denosumab [Xgeva]), as recommended by the American Society of Clinical Oncology (ASCO), should also be considered for patients with bone metastases.[27] Palliative radiation therapy may be utilized for local control of disease and to alleviate pain. Early involvement of palliative care teams is also recommended in the management of patients with metastatic disease, in particular when the disease is symptomatic or prognosis is deemed poor.
Conclusion
For the majority of postmenopausal patients with HR-positive metastatic breast cancer, we recommend a third-generation AI as first-line endocrine therapy. It is generally believed that the efficacy of the non-steroidal and steroidal AIs is equivalent, and these may be used interchangeably. The use of exemestane could, however, be deferred early on to allow for later use of the exemestane/everolimus combination when deemed clinically appropriate. A combination approach (anastrozole plus fulvestrant) may also be employed in the first-line setting, based on the SWOG S0226 trial. Depending on the agent used for first-line therapy, we suggest that second-line therapy and beyond can include an AI from a different class, tamoxifen, or fulvestrant. Ongoing investigation aims to identify strategies to overcome resistance to hormonal agents in an effort to improve outcomes for this patient population and to delay the time to chemotherapy use. Novel strategies should ideally be developed alongside predictive biomarkers of response to therapy, in order to minimize toxicity and maximize therapeutic benefit. Patient enrollment in clinical trials should be considered with every treatment decision.
Financial Disclosure:Dr. Connolly has received research funding from Novartis. Dr. Stearns has received research funding from Novartis and Pfizer.
References:
1. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784-95.
2. Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev. 2003;(2):CD002747.
3. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-84.
4. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299-309.
5. Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530-5.
6. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594-600.
7. Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18:3758-67.
8. Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101-9.
9. Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-70.
10. Mauri D, Pavlidis N, Polyzos NP, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-91.
11. Gibson L, Lawrence D, Dawson C, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;(4)CD003370.
12. Robertson JF, Lindemann JP, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized 'FIRST' study. Breast Cancer Res Treat. 2012;136:503-11.
13. Rose C, Vtoraya O, Pluzanska A, et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer. Comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer. 2003;39:2318-27.
14. Campos SM, Guastalla JP, Subar M, et al. A comparative study of exemestane versus anastrozole in patients with postmenopausal breast cancer with visceral metastases. Clin Breast Cancer. 2009;9:39-44.
15. Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774-80.
16. Mehta R, Barlow W, Albain K, et al. A phase III randomized trial of anastrozole versus anastrozole and fulvestrant as first-line therapy for postmenopausal women with metastatic breast cancer: SWOG S0226. Cancer Res. 2011;71:95A.
17. Bergh J, J̦nsson PE, Lidbrink E, et al. First results from FACT Рan open-label, randomized phase III study investigating loading dose of fulvestrant combined with anastrozole versus anastrozole at first relapse in hormone receptor positive breast cancer. Cancer Res. 2009;69(24 Suppl):Abstr 23.
18. Mohla S, Stearns V, Sathyamoorthy N, et al. The biology of hormone refractory breast and prostate cancer: an NCI workshop report. Cancer Biol Ther. 2009;8:1975-85.
19. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-9.
20. Novartis. Phase III study of BKM120/placebo with fulvestrant in postmenopausal patients with hormone receptor positive HER2-negative locally advanced or metastatic breast cancer refractory to aromatase inhibitor (BELLE-2). NCI trial ID: NCT01610284. Available from: http://clinicaltrials.gov/show/NCT01610284.
21. Finn RS, Crown JP, Lang I, et al. Results of a randomized phase 2 study of PD 0332991, a cyclin-dependent kinase (CDK) 4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+, HER2- advanced breast cancer (TRIO-18). Presented at the 2012 CTRC-AACR San Antonio Breast Cancer Symposium. December 4–8. San Antonio, TX. 2012. Abstr S1-6.
22. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77.
23. Pfizer. A study of PD-0332991 + letrozole vs. letrozole for 1st line treatment of postmenopausal women with ER+/HER2− advanced breast cancer. NCI trial ID: NCT0174027. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01740427?term=PD0332991+and+letrozole&rank=2.
24. Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013 May 6. [Epub ahead of print]
25. Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study.
J Clin Oncol. 2009;27:5529-37.
26. Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538-46.
27. Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221-7.