The therapeutic landscape for cutaneous melanoma has dramatically advanced in the last several years with the development, validation, and approval by the US Food and Drug Administration of several new therapies that have proven effective in treating metastatic disease. Considerable effort has been put into identifying prognostic and predictive markers of therapeutic response to better delineate the patient populations most likely to benefit from treatment. Baseline tumor burden has been described as a common clinical factor associated with treatment response: lower tumor burden at the time of therapeutic intervention is associated with improved responses and survival outcomes on several therapies. Some therapies have shown efficacy as adjuvant interventions in patients with subclinical disease following definitive treatment, further supporting their role in patients with minimal tumor burden. The increasing evidence that patients with lower tumor burden may be the ones who derive maximal benefit from several melanoma-directed therapies points toward the critical need for risk-tailored surveillance to permit early identification of melanoma metastasis in patients at high risk for recurrence.
Introduction
Over the past decade, the availability of effective therapies for cutaneous melanoma has expanded greatly. The US Food and Drug Administration (FDA) has approved new classes of therapeutic agents in both the adjuvant and metastatic settings, and there are numerous ongoing clinical trials. These therapies, which include drugs targeting specific mutant proteins and others that manipulate the innate immune system, have demonstrated substantial improvements in response rates and overall survival (OS) for melanoma patients, who previously had few options. Nevertheless, some patients respond poorly or not at all. Thus, there is a need to identify biomarkers and/or clinical features predictive of response.
Baseline disease burden has been shown to be associated with therapeutic responses and outcomes. Although tumor burden can be measured and reported in several ways (ie, tumor diameter [largest or combined], tumor volume, number of metastases), patients who have smaller and fewer metastases have improved clinical and pathologic responses and survival compared with patients with greater baseline tumor burden across multiple cancer types. These cancer types include uveal melanoma,[1-6] renal cell carcinoma,[7] breast cancer,[8-10] and Hodgkin,[11] and non-Hodgkin lymphoma,[12] and non–small-cell lung cancer (NSCLC).[13] Several liver-directed treatments for uveal melanoma, including surgical resection, chemoembolization, radioembolization, and percutaneous hepatic perfusion, elicit better responses and prolong progression-free survival (PFS) and OS with smaller baseline tumor burden.[1-6,14,15] Together, these studies support the significant impact of baseline tumor burden on clinical outcomes.
In cutaneous melanoma, a growing body of evidence describes the importance of tumor burden in treatment response, and it has been suggested that this clinical feature could help inform treatment decision making.[16] Thus, tumor burden could have implications for future clinical staging or national guidelines, since it is not accounted for in the current American Joint Committee on Cancer (AJCC) M staging for stage IV disease.[17] It logically follows that to exploit lower tumor burden to improve treatment outcomes, surveillance in certain patient groups becomes critical. In this article, we review the role of baseline tumor size in response and survival on traditional and contemporary therapies for the treatment of locoregional and distant melanoma recurrences. We also discuss the implications of these findings for surveillance strategies in high-risk melanoma patients to facilitate early detection of asymptomatic melanoma recurrences amenable to treatment.
Impact of Locoregional Melanoma Tumor Burden on Tumor Response and Overall Survival With Regional Therapies
Patients with unresectable locoregional recurrences or in-transit melanoma, in the absence of widespread distant metastatic disease, have improved survival compared with patients with systemic disease. This is reflected in TNM staging, which has a different and successively worse prognosis for M1a (skin or nonregional lymph node with normal lactate dehydrogenase [LDH] level), M1b (lung metastases with normal LDH level), and M1c (visceral metastases or elevated LDH level) stage IV melanoma. Even more favorable M1a disease can cause debilitating symptoms such as ulceration, pain, and bleeding that affect quality of life. M1a patients frequently experience recurrences, which can be challenging to manage, and there is also the substantial risk of distant metastasis.
These lesions can be treated by surgical resection in limited disease settings or with regional and/or systemic therapies, which can provide symptom alleviation and disease control. Such regional treatments include isolated limb infusion (ILI) and isolated limb perfusion (ILP) for unresectable and/or in-transit lesions isolated to the extremities, and talimogene laherparepvec (T-VEC), which also can have beneficial effects on uninjected lesions and low-volume distant metastases.
Percutaneous ILI can be used to treat melanoma of the extremities by isolating the limb from the systemic circulation, permitting the use of high-dose chemotherapy. Tumor burden has been shown to be a significant predictor of response to ILI. Steinman et al evaluated factors associated with ILI response, OS, and limb salvage.[18] Low tumor burden was defined as fewer than 10 lesions, with no lesion greater than 3 cm, while high tumor burden was defined as 10 lesions or more, or 1 lesion greater than 3 cm. Fifteen out of 23 patients (65%) with low tumor burden had a significant response (complete response [CR], or partial response [PR]), whereas only 6 out of 32 patients (19%) with high tumor burden had a significant response (P < .0001). Ten out of 14 patients (71%) who had a CR had low tumor burden. Patients exhibiting a CR had an improved 5-year survival rate (91%) compared with those who had less than a CR (34%; P = .011). Similarly, patients with low tumor burden had a significantly improved 5-year OS rate compared with patients with high tumor burden (69% and 29%, respectively; P = .007). A separate study of ILP in 91 patients at the National Cancer Institute also concluded that ILP may be most effective if utilized early when disease burden is low.[19]
T-VEC is an oncolytic viral immunotherapy that is FDA-approved for the treatment of unresectable stage IIIB–IV melanoma.[20,21] In a phase III trial in 436 patients, significantly more patients treated with T-VEC had durable and overall responses compared with those treated with granulocyte-macrophage colony-stimulating factor (GM-CSF). Furthermore, 40% of patients had a CR following T-VEC treatment. Of 414 patients with evaluable baseline tumor burden, those with tumor burden less than the median had significantly improved durable and overall response rates, as well as improved OS.[21] This was true in the T-VEC–alone arm and in the combined T-VEC and GM-CSF arm. In the T-VEC arm, the hazard ratio (HR) for death associated with lower tumor burden was 0.27, with a 43% mortality rate in patients with lower tumor burden vs 85% in patients with tumor burden greater than the median (P < .001). The authors suggested that tumor burden should be considered for prognostication and response prediction in future trials of T-VEC and other therapies.
Although there is recognition of tumor burden within AJCC substaging for regional disease, it is limited to nodal burden. These studies demonstrate the limitations of AJCC staging for predicting response to locoregional therapies and show that tumor burden in this setting is not merely a surrogate for staging. AJCC stage was not predictive of response to ILI [18] and was not an independent predictor of in-field PFS or OS with ILP.[19] With T-VEC, patients with lower tumor burden had improved OS when stages IIIB/IIIC/IVM1a and IVM1b/c were evaluated separately. Tumor burden was still a significant independent predictor of overall response, durable response, and OS when AJCC stage was included in multivariate analysis.[21]
Impact of Metastatic Melanoma Tumor Burden on Tumor Response and Overall Survival With Systemic Therapies
The prevalence of BRAF V600E/K mutations in melanoma led to the development of targeted agents to inhibit the constitutively active, mutant BRAF kinase and downstream signaling pathway. These therapies include BRAF inhibitors such as dabrafenib and vemurafenib and MEK inhibitors such as trametinib and cobimetinib. BRAF and MEK inhibitors are usually used in combination.
Prolonged survival and improved responses to BRAF and MEK inhibitors have been demonstrated in patients with a smaller metastatic disease burden.[22,23] In a landmark analysis of the phase III COMBI-d trial of dabrafenib and trametinib, the number of metastatic organ sites combined with LDH levels were identified as important prognostic factors for combination therapy.[22] The 3-year PFS rate for patients receiving combination therapy who had fewer than three metastatic organ sites and normal LDH levels was 38% compared with 27% for those with normal LDH levels alone. Rates of OS for patients receiving combination therapy were 62% for those with fewer than three metastatic organ sites and normal LDH and 54% for those with normal LDH levels alone. Nearly half (49%) of the patients with fewer than three involved organ sites and normal LDH levels who received combination therapy were alive after 3 years. The 5-year landmark analysis of part C of the BRF113220 trial, which represents the longest follow-up time on combination therapy, also found that patients with normal LDH levels and fewer than three involved organ sites derived the most benefit from dabrafenib/trametinib treatment, with 5-year PFS and OS rates of 25% and 51%, respectively.[24] Furthermore, a pooled analysis of phase III trials found that normal LDH level, fewer than three metastatic organ sites, and a sum of lesion diameters less than 66 mm identified the best prognostic group of those receiving combination therapy, with a 3-year PFS rate of 42%.[23]
It could be argued that these reported PFS/OS benefits of targeted therapies in patients with lower tumor burden are a result of lead-time bias. However, evalution of the individual responses of metastases shows that smaller metastases are more likely to have a CR to BRAF/MEK inhibition than are larger metastases.[25] A total of 24 patients collectively had 135 metastases (median, 4.5 per patient), with a median diameter of 16 mm (range, 5–108 mm). A CR occurred in 71 metastases (56.2%), including 42% of the metastases that had a baseline diameter greater than 10 mm. Metastases that exhibited a CR had a median diameter of 11 mm, as compared with a median diameter of 20 mm in metastases that exhibited a PR, were stable, or progressed (P < .001). With the exception of metastases within the lymph nodes, metastases with CRs at all other sites were significantly smaller than those with less than a CR. Of the 71 metastases with a CR, only 1 progressed.[25] The biology underpinning the durable response in smaller lesions remains incompletely understood. It is possible that smaller tumors allow better drug delivery into the tumor[26] or have accumulated less molecular heterogeneity and thus are more amenable to treatment. Although smaller individual metastases were associated with CRs, only 17% of patients had an overall CR and 75% had an overall PR. Thus, PFS was not associated with baseline tumor burden in this study.
Immunotherapies for melanoma and other cancers manipulate the patient’s own immune system to attack tumor cells. As with targeted therapies, responses and survival outcomes with cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) inhibitors are improved in patients who have lower tumor burden. In 59 individual, measurable metastases in 21 patients who received ipilimumab and bevacizumab in a phase I trial, smaller baseline tumor diameter was significantly predictive of improved PFS and OS.[27] Patients with tumor diameter at or below the median (38 mm) had a median PFS of 27.5 months and the median was not reached for OS. Conversely, patients with tumor diameters greater than 38 mm had a median PFS of 4.1 months and median OS of 12.6 months. With every 5-mm increase in baseline diameter, the risk for progression and death increased by 14% and 18%, respectively.
The response of individual metastases to the PD-1 inhibitor pembrolizumab has also been evaluated. A single-center analysis of patients enrolled in the phase I KEYNOTE-001 trial of pembrolizumab evaluated 442 metastases in 27 patients.[28] Approximately half of the metastases exhibited a CR, and 81% of patients had at least one metastasis that achieved a CR. Similar to the findings of a separate single-center study in 37 patients,[29] metastases that exhibited a CR were significantly smaller than metastases that did not exhibit a CR.[28] Progression after a CR was observed in only one metastasis. It is possible that additional biological factors related to pembrolizumab response may contribute to the prevention of progression in these small metastases.
Larger analyses of patients within KEYNOTE-001 have demonstrated that baseline disease burden is significantly associated with clinical response and outcomes in patients treated with pembrolizumab. Stratifying by median baseline tumor size (10.2 cm) in 583 patients, baseline tumor burden at or below the median was associated with objective response rate and OS in univariate analysis. In multivariate analysis, baseline tumor burden at or below the median was an independent predictor of OS (HR, 0.61; P < .001).[30] In the subset of patients for whom pembrolizumab was first-line treatment, 83% of patients with baseline tumor burden at or below the median were alive after 1 year, compared with only 56% of patients with baseline tumor burden greater than the median (P < .001). A separate report of a pooled analysis of 581 patients with measurable disease from eight cohorts within the KEYNOTE-001 trial had similar findings. Baseline tumor size less than the median (also 10.2 cm) had an objective response rate (CR + PR) of 42.8%, whereas patients with tumor burden at or above the median had an objective response rate of 24.1%.[31] In a recent long-term analysis of KEYNOTE-001 complete responders, tumor burden was significantly associated with CR, and CR rates were higher in patients with smaller baseline tumor burden, particularly when coupled with positive programmed death ligand 1 (PD-L1) expression. CR rates were 37.6% in patients with small (1.0–4.9 cm), 17.8% with medium (5.0–9.9 cm), and 4.7% with large (10–90 cm) baseline tumor burden.[32] Together, these subgroup analyses show that, although patients with higher tumor burden can still benefit from pembrolizumab, improved antitumor activity is seen in patients with low tumor burden.
Mechanistically, it has been reported that the ratio of T-cell invigoration to tumor burden may be associated with anti–PD-1 response.[33] Using pretreatment and posttreatment peripheral blood from 29 stage IV melanoma patients receiving pembrolizumab, immune profiling was performed to investigate pharmacodynamic changes in circulating exhausted-phenotype CD8+ T cells (Tex cells). Despite having immunologic responses, some patients did not have objective responses. The researchers found that the ratio of Tex-cell reinvigoration to tumor burden distinguished clinical outcomes and predicted response. Therefore, they concluded that the relative balance between tumor burden and the amount of T-cell reinvigoration, not just T-cell reinvigoration alone, contributes to response. This raises the possibility that even robust reinvigoration by anti–PD-1 therapy may be clinically ineffective if the tumor burden is too high. These findings may represent the first steps in understanding the mechanisms behind the different response rates seen in the pembrolizumab clinical trials described previously.
Impact of Adjuvant Therapy on Minimal Residual Disease After Definitive Melanoma Treatment
Historically, stage III patients have had a substantial risk of relapse, with 55% to 60% of patients experiencing disease progression within 4 years despite complete surgical resection and adjuvant interferon.[34] Thus, there certainly was and is an ongoing clinical need for effective adjuvant therapies for high-risk stage III patients. Several therapies proven to be beneficial in the metastatic setting have now been evaluated in the adjuvant setting.
The combination of dabrafenib and trametinib was recently approved by the FDA for adjuvant melanoma treatment.[35] In an interim analysis of the double-blind, placebo-controlled, phase III COMBI-AD trial, 870 patients with stage IIIA–IIIC (AJCC 7th edition) melanoma harboring BRAF V600E or V600K mutations were randomized to dabrafenib plus trametinib or placebo for 12 months. With a median follow-up of 2.8 years, the respective estimated 3-year relapse-free survival (RFS) and OS rates were 58% and 86% in the dabrafenib/trametinib group compared with 39% (P < .001) and 77% (P = .0006) in the placebo group. The P value for OS was not below the prespecified, interim-analysis significance, but follow-up is ongoing, with additional interim analyses planned before the estimated study completion in 2023.
Like targeted therapies, immunotherapies have also been validated as treatment strategies in melanoma with residual microscopic, clinically occult disease. High-dose ipilimumab (10 mg/kg) was approved in 2015 as adjuvant therapy for stage III patients at high risk for recurrence after complete resection. This approval was based on improvements in RFS, distant metastasis–free survival (DMFS), and OS on ipilimumab compared with placebo.[36,37] With a median follow-up of 5.3 years, RFS was 40.8% with ipilimumab and 30.3% with placebo (P < .001). DMFS was 48.3% with ipilimumab and 38.9% with placebo (P = .002), and OS rates were 65.4% with ipilimumab and 54.4% with placebo (P = .001).[36] Ipilimumab demonstrated benefits for patients with microscopic or macroscopic nodal disease, as well as for patients without an ulcerated primary melanoma.[37]
In the CheckMate-238 trial, adjuvant treatment (for up to 1 year or until disease recurrence) with the PD-1 inhibitor nivolumab was compared with ipilimumab in patients with stage IIIB, IIIC, and IV melanoma undergoing complete resection.[38] RFS was 66.4% vs 52.7% (P < .001) for nivolumab vs ipilimumab treatment. This significant benefit of nivolumab over ipilimumab persisted even in subanalyses accounting for stage, ulceration, nodal tumor burden, PD-L1 expression, and BRAF mutation. Similarly, KEYNOTE-054 demonstrated that adjuvant pembrolizumab improves RFS for stage III patients, including stage IIIA patients with greater than 1 mm of nodal tumor burden-with 1-year recurrence rates of 75.4% vs 61.0% for pembrolizumab-treated patients compared with those who received placebo (P < .001).[39] Despite the potential toxicities associated with adjuvant targeted therapies and immunotherapies, these trials confirm their efficacy in patients without clinically detectable cancer who still have subclinical microscopic residual disease following definitive surgery.
Surveillance of Patients With Melanoma Following Definitive Therapy Who Are at High Risk for Recurrence
Although the intrinsic biology of responsive low tumor burden remains to be understood, one way to leverage lower tumor burden for optimal treatment response is to identify patients’ recurrences and metastases early through asymptomatic surveillance imaging. There has been significant debate over the role of surveillance imaging in patients with a history of melanoma but no radiographic evidence of active disease. Many early studies conducted prior to the modern era of therapy were unable to show meaningful OS benefits that justified the costs of surveillance imaging.[40-43] However, some larger studies in the pre-immunotherapy era did identify a significant subset of patients who could potentially benefit from modern therapy for asymptomatic metastatic disease. In a large 2003 study, chest x-ray and visceral ultrasonography were not useful, but some patients underwent CT imaging (to confirm suspicious findings) or lymph node sonography, and the latter identified 77% of detected recurrences at an early stage.[44]
Other studies have suggested that patients, not imaging, are most likely to identify recurrences.[45-48] Many of these studies did not include standardized imaging as a part of surveillance, however, leaving detection primarily to the patient or clinical examination. Given current improvements in treatment outcomes in patients with lower tumor burden and recent adjuvant therapy advances, the goal of surveillance imaging should be to identify patients with asymptomatic, minimal tumor burden that can be treated and is unlikely to be detected by the patient or through clinical examination.
Recently, several studies have demonstrated that routine cross-sectional imaging can identify the majority of asymptomatic distant recurrences amenable to therapeutic intervention.[49-53] In a cohort of patients with resected stage II–IV melanoma treated at the National Cancer Institute in adjuvant immunotherapy trials, patients underwent standard CT-imaging follow-up at 6-month intervals during the first 2 years and then annually.[51] Of the 466 patients, 225 (48%) developed recurrence during the 5-year observation period. CT imaging identified the majority (59%) of tumor recurrences, compared with physical examination (27%) or laboratory studies (14%). In patients with systemic recurrence, 75% were detected by CT imaging. This was the case regardless of patients’ stage at enrollment.
Lead-time bias is a concern when interpreting results of surveillance imaging. In diseases with slow progression, such as early-stage prostate cancer, lead-time bias can significantly impact survival analysis. Melanoma, however, is a more aggressive disease than prostate cancer. In a study of 1,969 patients undergoing melanoma surveillance in the era before contemporary therapy, a factual gain was observed in survival time for the detection of metastasis in an early phase of development (based on tumor burden and/or potential for curative intent surgery) beyond lead time bias.[49]
KEY POINTS
- Contemporary melanoma therapies have significantly improved outcomes for patients in the adjuvant setting and for those with advanced disease.
- Lower tumor burden is a clinical feature that has been associated with improved responses and survival outcomes across multiple therapies in melanoma.
- Identification of high-risk patients in order to provide appropriate surveillance to facilitate early detection of melanoma recurrences is important to leverage the benefits of lower tumor burden in treatment.
When considering routine surveillance imaging to facilitate early detection of small tumor burden, clinicians need to be selective for patients who have the highest risk of recurrence. What is considered high risk needs to be further clarified, given that a subset of patients who will progress to metastatic melanoma are diagnosed with seemingly low-risk disease (by clinicopathologic staging) that does not currently qualify for surveillance imaging. Although loose surveillance imaging guidelines are in place for patients with stage IIB/IIC/III disease (consider imaging every 3–12 months, category 2B), there is no such recommendation for the majority of patients, namely those with stage I and IIA disease. High-risk, early-stage (I–IA) melanoma remains an area with unmet clinical need. A recent study found that thin melanomas (< 2 mm) accounted for more than twice as many deaths from melanoma as thick (> 4 mm) melanomas in the 1990–2009 Queensland Cancer Registry. Similar findings have been reported in the US SEER [Surveillance Epidemiology and End Result] database.[54,55] Expanding the role of disease monitoring in appropriate patients with early-stage melanoma may help detect occult metastatic disease prior to symptomatic recurrence.
The current AJCC staging guidelines (8th edition, 2017) note the relationship between accurate staging, contemporary melanoma treatment, and patient outcomes.[17] The guidelines propose that staging will continue to evolve to potentially incorporate other clinical, immune, and molecular features of the tumor. The use of molecular assays has contributed significantly to risk stratification in other cancers, including breast cancer, prostate cancer, and uveal melanoma, and have been recognized in national guidelines on the management of these diseases. Clinical application has been reported of a molecular gene expression profile test for cutaneous melanoma that has been both prospectively and independently validated for risk stratification of stage I–III patients.[56-59] Given that the test can identify patients at highest risk of recurrent disease, it may become an option for stratifying observation intensity for stage I/II patients and for making decisions regarding adjuvant therapies.
It has been argued that routine imaging is not cost-effective because of a low detection rate, but studies have previously assumed that all patients should have routine imaging, without first selecting those with high biological risk.[43,60,61] A 2014 study modeled with data from 1992 to 2007 at MD Anderson Cancer Center, which was limited to the identification of surgically resectable recurrences,[60] could now be expanded, given the availability of better systemic treatments. More recently, a prospective study in 290 stage IIB–IIIC patients showed that biannual CT scan and brain MRI for the first 5 years after diagnosis identified 84% of distant metastases, primarily in an asymptomatic state.[52] In a cost analysis of the same cohort, biannual CT scan was found to be cost-effective for stage IIB patients up to 3 years and up to 5 years for stage IIC/III patients based on incremental cost-effectiveness ratios.[62] Another consideration with respect to the cost/benefit ratio of early detection–identified treatment-amenable disease is that end-of-life care has been estimated to constitute a substantial proportion of melanoma-related healthcare costs.[63] Given the long-term response durability with newer therapies and the interaction of lower tumor burden with response, the paradigm of what is considered cost-effective care may be shifting and is deserving of additional studies.
Conclusions and Future Perspectives
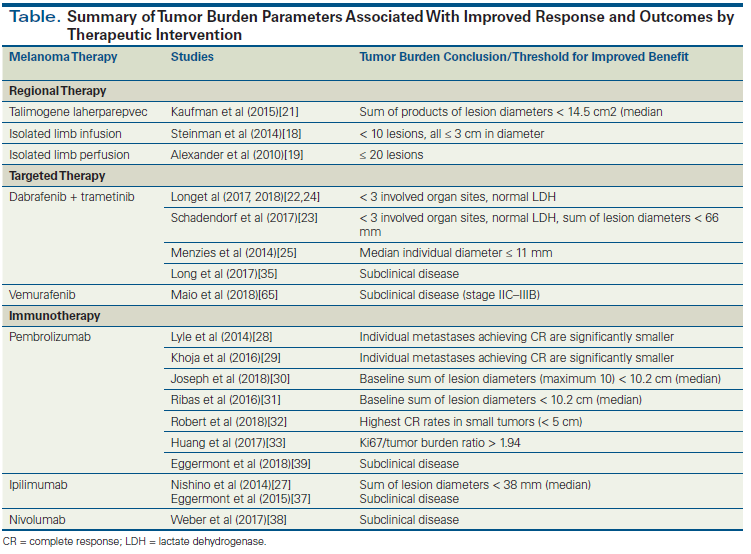
In the era of modern immunotherapy and targeted therapy, patients have much greater odds of a successful outcome for the treatment of their disease. Median survival times are vastly improved over those of prior decades, and clinical trials offer great hope for even further therapeutic advances. Durable responses have been observed with targeted therapy and immunotherapy.[24,64] Of patients who completed 2 years of pembrolizumab, 86% had durable responses lasting almost 2 years after treatment discontinuation.[64] Despite these significant advances, however, many patients still succumb to metastatic disease. A greater understanding of the role of tumor burden as a modulating factor in the efficacy of antimelanoma therapy is important. It is apparent that the current available treatments have greater odds of efficacy in patients with a smaller, more-limited disease burden (Table). Whereas emerging evidence suggests that the balance between tumor burden and the amount of T-cell reinvigoration plays a role in the anti–PD-1 response, the mechanistic relationship between tumor burden and response to other treatments remains to be fully elucidated. Additionally, a standardized means of measuring and reporting tumor burden could be useful to further prospectively analyze the predictive role of tumor burden and to improve its utility across studies and in the clinic. Expansion of the role of molecular testing for risk stratification may help identify subgroups of patients at highest risk for relapse and in need of higher-intensity surveillance, sparing many low-risk patients the need for surveillance. Taken together, the existing evidence on tumor burden and treatment outcomes supports further exploration of tumor burden as a predictive factor for future clinical trials and potential consideration in clinical practice when evaluating therapy choice and discussing response expectations with individual patients.
Financial Disclosure: Dr. Carvajal has been a consultant to AstraZeneca, Bristol-Myers Squibb, Iconic Therapeutics, Janssen, Merck, Novartis, and Roche/Genentech; and an advisory board member for Aura Biosciences, Castle Biosciences, Chimeron, and Rgenix. Dr. Poklepovic has been a consultant to Bristol-Myers Squibb and is an advisory board member for Castle Biosciences. Kristen Meldi Plasseraud, PhD, an employee and option holder at Castle Biosciences, Inc, provided writing support for this review.
References:
1. Frenkel S, Nir I, Hendler K, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93:1042-6.
2. Gonsalves CF, Eschelman DJ, Sullivan KL, et al. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196:468-73.
3. Gupta S, Bedikian AY, Ahrar J, et al. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: response, survival, and prognostic factors. Am J Clin Oncol. 2010;33:474-80.
4. Hsueh EC, Essner R, Foshag LJ, et al. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100:122-9.
5. Huppert PE, Fierlbeck G, Pereira P, et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol. 2010;74:e38-44.
6. Leyvraz S, Spataro V, Bauer J, et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol. 1997;15:2589-95.
7. Iacovelli R, Lanoy E, Albiges L, Escudier B. Tumour burden is an independent prognostic factor in metastatic renal cell carcinoma. BJU Int. 2012;110:1747-53.
8. Caudle AS, Gonzalez-Angulo AM, Hunt KK, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:1821-8.
9. Fei F, Messina C, Slaets L, et al. Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase III trial. Eur J Cancer. 2015;51:301-9.
10. Montagna E, Bagnardi V, Rotmensz N, et al. Pathological complete response after preoperative systemic therapy and outcome: relevance of clinical and biologic baseline features. Breast Cancer Res Treat. 2010;124:689-99.
11. Gobbi PG, Valentino F, Bassi E, et al. Chemoresistance as a function of the pretherapy tumor burden and the chemotherapy regimen administered: differences observed with 2 current chemotherapy regimens for advanced Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2011;11:396-402.
12. Jagannath S, Velasquez WS, Tucker SL, et al. Tumor burden assessment and its implication for a prognostic model in advanced diffuse large-cell lymphoma. J Clin Oncol. 1986;4:859-65.
13. Wang Y, Sung C, Dartois C, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167-74.
14. Akyuz M, Yazici P, Dural C, et al. Laparoscopic management of liver metastases from uveal melanoma. Surg Endosc. 2016;30:2567-71.
15. Abbott AM, Doepker MP, Kim Y, et al. Hepatic progression-free and overall survival after regional therapy to the liver for metastatic melanoma. Am J Clin Oncol. 2018;41:747-53.
16. Warner AB, Postow MA. Bigger is not always better: tumor size and prognosis in advanced melanoma. Clin Cancer Res. 2018 Jun 14. [Epub ahead of print]
17. Gershenwald JD, Scoyler RA, Hesss KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;6:472-92.
18. Steinman J, Ariyan C, Rafferty B, Brady MS. Factors associated with response, survival, and limb salvage in patients undergoing isolated limb infusion. J Surg Oncol. 2014;109:405-9.
19. Alexander HR Jr, Fraker DL, Bartlett DL, et al. Analysis of factors influencing outcome in patients with in-transit malignant melanoma undergoing isolated limb perfusion using modern treatment parameters. J Clin Oncol. 2010;28:114-8.
20. Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780-8.
21. Kaufman H, Amatruda T, Nemunaitis JJ, et al. Tumor size and clinical outcomes in melanoma patients (MEL pts) treated with talimogene laherparepvec (T-VEC). J Clin Oncol. 2015;33:9074.
22. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-9.
23. Schadendorf D, Long GV, Stroiakovski D, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. 2017;82:45-55.
24. Long GV, Eroglu Z, Infante J, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2018;36:667-73.
25. Menzies AM, Haydu LE, Carlino MS, et al. Inter- and intra-patient heterogeneity of response and progression to targeted therapy in metastatic melanoma. PLoS One. 2014;9:e85004.
26. Sefidgar M, Soltani M, Raahemifar K, et al. Effect of tumor shape, size, and tissue transport properties on drug delivery to solid tumors. J Biol Eng. 2014;8:12.
27. Nishino M, Giobbie-Hurder A, Ramaiya NH, Hodi FS. Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J Immunother Cancer. 2014;2:40.
28. Lyle MK, Lee JHJ, Menzies AM, et al. Lesion-specific patterns of response and progression with anti-PD1 treatment in metastatic melanoma (MM). J Clin Oncol. 2014;32:9077.
29. Khoja L, Kibiro M, Metser U, et al. Patterns of response to anti-PD-1 treatment: An exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients. Br J Cancer. 2016;115:1186-92.
30. Joseph RW, Elassaiss-Schaap J, Kefford RF, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018 Apr. 23. [Epub ahead of print]
31. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-9.
32. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-74.
33. Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60-5.
34. Eggermont AM, Suciu S, Rutkowski P, et al. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: Ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer. 2016;55:111-21.
35. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813-23.
36. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845-55.
37. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-30.
38. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824-35.
39. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789-801.
40. Meyers MO, Yeh JJ, Frank J, et al. Method of detection of initial recurrence of stage II/III cutaneous melanoma: analysis of the utility of follow-up staging. Ann Surg Oncol. 2009;16:941-7.
41. Weiss M, Loprinzi CL, Creagan ET, et al. Utility of follow-up tests for detecting recurrent disease in patients with malignant melanomas. JAMA. 1995;274:1703-5.
42. Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance. Patient or physician based? Ann Surg. 1995;221:566-9; discussion 569-71.
43. Bassères N, Grob JJ, Richard MA, et al. Cost-effectiveness of surveillance of stage I melanoma. A retrospective appraisal based on a 10-year experience in a dermatology department in France. Dermatology. 1995;191:199-203.
44. Garbe C, Paul A, Kohler-SpÓth H, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. J Clin Oncol. 2003;21:520-9.
45. Berger AC, Ollila DW, Christopher A, et al. Patient symptoms are the most frequent indicators of recurrence in patients with American Joint Committee on Cancer stage II melanoma. J Am Coll Surg. 2017;224:652-9.
46. Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28:3042-7.
47. Lee AY, Droppelmann N, Panageas KS, et al. Patterns and timing of initial relapse in pathologic stage II melanoma patients. Ann Surg Oncol. 2017;24:939-46.
48. Damude S, Hoekstra-Weebers JE, Francken AB, et al. The MELFO-Study: Prospective, randomized, clinical trial for the evaluation of a stage-adjusted reduced follow-up schedule in cutaneous melanoma patients-results after 1 year. Ann Surg Oncol. 2016;23:2762-71.
49. Leon-Ferre RA, Kottschade LA, Block MS, et al. Association between the use of surveillance PET/CT and the detection of potentially salvageable occult recurrences among patients with resected high-risk melanoma. Melanoma Res. 2017;27:335-41.
50. Leiter U, Buettner PG, Eigentler TK, et al. Is detection of melanoma metastasis during surveillance in an early phase of development associated with a survival benefit? Melanoma Res. 2010;20:240-6.
51. Park TS, Phan GQ, Yang JC, et al. Routine computer tomography imaging for the detection of recurrences in high-risk melanoma patients. Ann Surg Oncol. 2017;24:947-51.
52. Podlipnik S, Carrera C, Sanchez M, et al. Performance of diagnostic tests in an intensive follow-up protocol for patients with American Joint Committee on Cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: a prospective cohort study. J Am Acad Dermatol. 2016;75:516-24.
53. Madu MF, Timmerman P, Wouters MWJM, et al. PET/CT surveillance detects asymptomatic recurrences in stage IIIB and IIIC melanoma patients: a prospective cohort study. Melanoma Res. 2017;27:251-7.
54. Whiteman DC, Baade PD, Olsen CM. More people die from thin melanomas (< 1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J Invest Dermatol. 2015;135:1190-3.
55. Criscione VD, Weinstock MA. Melanoma thickness trends in the United States, 1988-2006. J Invest Dermatol. 2010;130:793-7.
56. Hsueh EC, DeBloom JR, Lee J, et al. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J Hematol Oncol. 2017;10:152.
57. Zager JS, Gastman BR, Leachman S, et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer. 2018;18:130.
58. Schuitevoerder D, Heath M, Cook RW, et al. Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. J Drugs Dermatol. 2018;17:196-9.
59. Berger AC, Davidson RS, Poitras JK, et al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr Med Res Opin. 2016;32:1599-604.
60. Rueth NM, Xing Y, Chiang YJ, et al. Is surveillance imaging effective for detecting surgically treatable recurrences in patients with melanoma? A comparative analysis of stage-specific surveillance strategies. Ann Surg. 2014;259:1215-22.
61. Hofmann U, Szedlak M, Rittgen W, et al. Primary staging and follow-up in melanoma patients–monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87:151-7.
62. Podlipnik S, Moreno-Ramirez D, Carrera C, et al. Cost-effectiveness analysis of imaging strategy for an intensive follow-up of patients with AJCC stage IIB, IIC and III malignant melanoma. Br J Dermatol. 2018 Jun 7. [Epub ahead of print]
63. Guy GP Jr, Ekwueme DU, Tangka FK, Richardson LC. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-45.
64. Long GV, Schachter J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol. 2018;36:9503.
65. Maio M, Lewis K, Demidov L, et al. Adjuvant vemurafenib in resected, BRAFV600 mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018;19:510-20.