Promising Treatment To Raise the Standard of Care in Lower-Risk MDS-Related Anemia
This article is sponsored by Bristol Myers Squibb. Dr. Shah was compensated by BMS for his participation.
Myelodysplastic syndromes (MDS) are a group of hematologic malignancies in which a person’s bone marrow doesn’t produce enough healthy red blood cells, white blood cells or platelets.1 Current estimates suggest 20,000 people are diagnosed with MDS in the United States each year.2
If left untreated, MDS can progress and result in symptoms like anemia, defined by low red blood cell counts (i.e. hemoglobin levels below the normal range, less than 12 g/dL for women and less than 13.5 g/dL for men3), vulnerability to infection from low white blood cell counts, or risk of bleeding or bruising due to low platelet counts. As MDS progresses, there’s an added risk that it may transform into acute myeloid leukemia (AML), an aggressive form of blood cancer, making early detection and treatment crucial.2 There are also certain treatments available that are approved for use only after a person has been diagnosed with MDS. Anemia is a hallmark of the disease with ~90% of patients developing anemia due to their lower-risk MDS.1
Anemia Progression and Transfusions
Red blood cell (RBC) transfusions may become a necessary part of life as anemia progresses. Following the failure of other treatments for anemia, impacted individuals may require regular transfusions for years.4 Erythropoietin-stimulating agents, known as ESAs, are one treatment option that can increase the body’s natural production and release of RBCs, helping to reduce the frequency of transfusions.5
However, these therapies may not eliminate the need for transfusions, with many people with MDS continuing to rely on them to combat anemia.4 In some instances, treatment with RBC transfusions and ESAs may become less effective over time, putting these individuals at greater risk for poor overall survival.6
Not a One-Size-Fits-All Approach
Given the challenges of treating MDS-related anemia, it’s important for doctors to regularly monitor their patients experiencing anemia for disease progression. Dr. Ashish Shah, a physician at Advanced Care Oncology and Hematology Associates (ACOHA) in New Jersey has over 15 years of experience treating hematological malignancies and MDS. He has seen, firsthand, how the MDS treatment landscape has evolved.
Dr. Ashish Shah

"The treatment of MDS and its symptoms is not one-size-fits-all, and we have to consider the specific circumstances and needs of each patient. Some can manage for many years without treatment while others may require intensive care early on,” said Dr. Shah. “ESAs have long been considered the standard of care option for those with lower-risk MDS but transfusion burden remains. Our goal as physicians is to improve quality of life, and patients have a better chance of achieving that with transfusion independence."
A Modern, First-Line Option
Reblozyl® (luspatercept-aamt) is an injectable prescription medicine approved in August 2023 as a first-line treatment for anemia in adults who are ESA-naïve with very low- to intermediate-risk MDS who may require regular RBC transfusions. Reblozyl is not indicated for use as a substitute for transfusions in patients who require immediate correction of their anemia.
In the Phase 3 open-label, randomized COMMANDS study7,8 Reblozyl was compared to an ESA option, epoetin alfa, in adult patients with MDS aged 18 years or older who required transfusion support. Results showed that Reblozyl achieved superior efficacy compared to epoetin alfa in the study’s primary endpoint of concurrent RBC transfusion independence for at least 12 weeks and hemoglobin increase – making Reblozyl the first and only therapy to demonstrate superior efficacy compared to an ESA in lower-risk MDS-related anemia.
The study evaluated transfusion independence and hemoglobin increase at both a 12-week and transfusion-free period. Key secondary endpoints observed include hematological improvement-erythroid increase of at least 8 weeks achieved by 74.1% of Reblozyl patients, RBC transfusion independence of at least 12 weeks and RBC transfusion independence of 24 weeks achieved by 66.7% and 47.6% of Reblozyl patients, respectively. Patients treated with Reblozyl were twice as likely to achieve both transfusion independence for 12 weeks and a 1.5 gram per deciliter increase in hemoglobin compared to those treated with epoetin alfa. Reblozyl-treated patients also demonstrated a numerically longer duration of transfusion freedom (2.5 years) compared to epoetin alfa-treated patients (1.5 years).
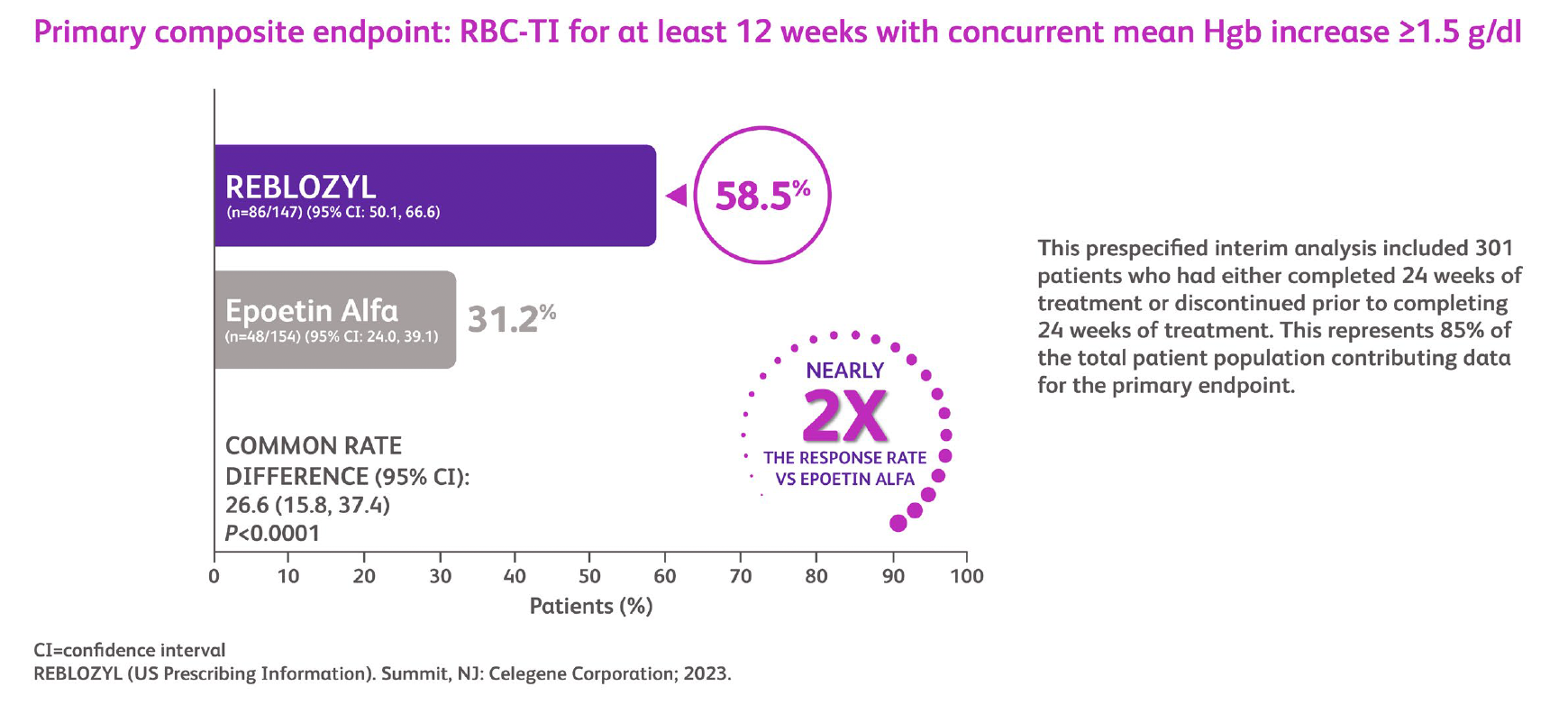
Transfusion Independence and Hgb Increase
“With Reblozyl, people with MDS have the potential to reduce the need for frequent transfusions earlier in their disease journey,” says Dr. Shah. “It’s a treatment modality that we use regularly with patients at my own practice and now consider it a first-line option. The goal remains to help patients go from transfusion dependent to transfusion independent.”
With treatment options like Reblozyl, people living with MDS may have a better chance to treat their anemia by increasing hemoglobin, reduce the frequency of red blood cell transfusions and improve their anemia. It’s important to talk to patients about treatment options, including Reblozyl, to determine which may be right for them as they navigate MDS-related anemia treatment.
The most common treatment-emergent adverse events observed in the COMMANDs trial in at least 10% of patients were diarrhea, fatigue, COVID-19, hypertension, dyspnea, nausea, peripheral edema, asthenia, dizziness, anemia, back pain and headache. Rates of reported fatigue and asthenia were shown to decrease over time. Please see additional Important Safety Information below.
For more information, visit www.reblozyl.com.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Thrombosis/Thromboembolism
In adult patients with beta thalassemia, thromboembolic events (TEE) were reported in 8/223 (3.6%) of REBLOZYL-treated patients. TEEs included deep vein thrombosis, pulmonary embolus, portal vein thrombosis, and ischemic stroke. Patients with known risk factors for thromboembolism (splenectomy or concomitant use of hormone replacement therapy) may be at further increased risk of thromboembolic conditions. Consider thromboprophylaxis in patients at increased risk of TEE. Monitor patients for signs and symptoms of thromboembolic events and institute treatment promptly.
Hypertension
Hypertension was reported in 11.4% (63/554) of REBLOZYL-treated patients. Across clinical studies, the incidence of Grade 3 to 4 hypertension ranged from 2% to 9.6%. In ESA-naïve adult patients with MDS with normal baseline blood pressure, 23 (36%) patients developed SBP≥140 mm Hg and 11 (6%) patients developed DBP ≥80 mm Hg. Monitor blood pressure prior to each administration. Manage new or exacerbations of preexisting hypertension using anti-hypertensive agents.
Embryo-Fetal Toxicity
REBLOZYL may cause fetal harm when administered to a pregnant woman. REBLOZYL caused increased post-implantation loss, decreased litter size, and an increased incidence of skeletal variations in pregnant rat and rabbit studies. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 3 months after the final dose.
ADVERSE REACTIONS
Grade ≥3 (≥2%) adverse reactions included hypertension and dyspnea.
The most common (≥10%) all-grade adverse reactions included diarrhea, fatigue, hypertension, peripheral edema, nausea, and dyspnea.
LACTATION
It is not known whether REBLOZYL is excreted into human milk or absorbed systemically after ingestion by a nursing infant. REBLOZYL was detected in milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because many drugs are excreted in human milk, and because of the unknown effects of REBLOZYL in infants, a decision should be made whether to discontinue nursing or to discontinue treatment. Because of the potential for serious adverse reactions in the breastfed child, breastfeeding is not recommended during treatment and for 3 months after the last dose.
DRUG ABUSE POTENTIAL
Abuse: Abuse of REBLOZYL may be seen in athletes for the effects on erythropoiesis. Misuse of drugs that increase erythropoiesis, such as REBLOZYL, by healthy persons may lead to polycythemia, which may be associated with life-threatening cardiovascular complications.
Please see US Full Prescribing Information and Patient Information, for REBLOZYL:
packageinserts.bms.com/pi/pi_reblozyl.pdf
REFERENCES
- Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Reviews. 2019;34:1-15. doi:10.1016/j.blre.2018.09.001.
- Myelodysplastic Sydrome (MDS) Research Funded by LLS. Leukemia & Lymphoma Society. https://www.lls.org/research/myelodysplastic-syndrome-mds-research-funded-lls. Accessed March 27, 2025
- Anemia. American Society of Hematology. https://www.hematology/org/education/patients/anemia. Accessed April 21, 2025
- Meunier M, Park S. Lower-risk myelodysplastic syndromes: Current treatment options for anemia. EJHaem. 2022. https://doi.org/10.1002/jha2.523.
- Platzbecker U, Kubasch AS, Homer-Bouthiette C, Prebet T. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia. 2021;35(8):2182–2198. doi:10.1038/s41375-021-01265-7.
- Germing, Ulrich et al. Treatment of Anemia in Transfusion-Dependent and Non-Transfusion-Dependent Lower-Risk MDS: Current and Emerging Strategies. HemaSphere 3(6):p e314, December 2019. doi:10.1097/HS9.0000000000000314
- Della Porta, Matteo Giovanni et al. Luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): primary analysis of a phase 3, open-label, randomised, controlled trial. Lancet. 2024. https://doi.org/10.1016/S2352-3026(24)00203-5.
- Reblozyl U.S. Prescribing Information. Available at: https://packageinserts.bms. com/pi/pi_reblozyl.pdf. Accessed March 27, 2025
REBLOZYL® is a trademark of Celgene Corporation, a Bristol Myers Squibb company.
REBLOZYL® is licensed from Merck & Co. Inc., Rahway, NJ, USA and its affiliates.
© 2025 Bristol-Myers Squibb Company.
[2007-US-2500118] [05/25]

2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.