Cancer progresses in a stepwise fashion. Oligometastatic cancer is an intermediate stage of tumor spread between localized disease and disseminated metastases. Oligometastatic prostate cancer is defined as up to five extrapelvic lesions on conventional imaging. There are controversies surrounding the management of this malignancy, but retrospective and population-based studies suggest a role for radical prostatectomy. Despite insufficient data to draw conclusions regarding the effectiveness of aggressive therapies on overall or cancer-specific survival of patients with oligometastatic prostate cancer, current studies suggest that surgery decreases tumor burden, disease-related morbidity, and the need for palliative surgical intervention, while increasing the period of time to development of castration-resistant disease.
Introduction
The dramatic stage migration of prostate cancer toward earlier-stage, lower-grade disease at diagnosis is due to the widespread use of prostate-specific antigen (PSA) screening. In the pre-PSA era, regional or distant metastatic disease was seen in 25% of patients at diagnosis; since the introduction of PSA screening, however, fewer than 5% of men with prostate cancer are diagnosed with synchronous metastatic disease.[1] The US Preventive Services Task Force recommendation against PSA-based prostate cancer screening has resulted in an increased incidence of patients with metastatic prostate cancer at diagnosis.[2-4]
The current standard of care for men with metastatic prostate cancer targets the androgen axis (by testosterone suppression using surgical or hormonal castration). The treatment of the primary tumor in the metastatic setting is limited to patients with significant local symptoms secondary to the primary tumor, and is undertaken only as a palliative measure.[5]
Men with metastatic hormone-sensitive prostate cancer are typically treated with immediate or deferred systemic androgen deprivation therapy (ADT), with or without antiandrogen agents.[6-9] The addition of chemotherapy to hormonal therapy in the management of metastatic hormone-sensitive prostate cancer has been shown to improve survival and highlights the potential benefit of a multimodal approach in treating men with metastatic disease.[10,11] The broad, nontargeted systemic treatment of metastatic prostate cancer patients irrespective of disease burden was challenged by findings from the CHAARTED, STAMPEDE, and GETUG-AFU 15 trials.[10-12] Furthermore, recently reported results of the LATITUDE trial demonstrated improvements in both overall survival (OS) and radiographic progression-free survival (PFS) following treatment with abiraterone (plus prednisone and ADT) across all subgroups of patients with metastatic prostate cancer-including those with visceral metastasis and more than 10 bone lesions-compared with men randomized to ADT plus placebo.[13] The findings of these trials suggest that chemohormonal therapy improved survival in men with high-volume disease, whereas this treatment failed to yield a survival benefit with long-term follow-up in men with low-volume or oligometastatic disease.[10-13]
Despite the benefits observed with some of the previously mentioned therapies, metastatic prostate cancer is associated with a 5-year survival rate of only 28%, and carries a large economic burden.[14,15] This suggests the existence of a distinct phenotype of metastatic disease that may warrant distinct treatment strategies compared with the approaches used for nonmetastatic disease. Historically, radical prostatectomy (RP) and radiation therapy (RT) have been offered only to men with localized prostate cancer, and recent studies indicate that these modalities demonstrate sustained oncologic benefits in the setting of locally advanced disease.[16-21] In contrast, very few data exist to support treatment of primary prostate cancer in the metastatic setting. While the body of evidence in support of achieving local disease control to improve both the rate of response to systemic therapy and survival is well established in other malignancies (including metastatic renal cell, colon, breast, and ovarian cancers, and glioblastoma), similar evidence is lacking in the medical literature on prostate cancer.[22-28]
Extrapolating from other malignancies to metastatic prostate cancer, there is growing interest in elucidating the role of cytoreductive prostatectomy in management of low-volume metastatic or oligometastatic prostate cancer. Many theories have been proposed in support of local disease control; the leading theory is that, despite systemic therapy, the primary tumor may continue to harbor viable tumor cells with lethal molecular features and serve as a source for the seeding of new metastatic foci.[29,30]
Despite a paucity of level 1 evidence to support treatment of the primary tumor in metastatic prostate cancer, emerging data suggest improved survival with this approach in men with metastatic prostate cancer and locally advanced prostate cancer (ie, lymph node–positive disease).[20,21,31-36] The management of oligometastatic prostate cancer is complex owing to variation in the definition of oligometastatic prostate cancer and inconsistency among published data in the literature. In this review, we will examine the theories supporting treatment to achieve local disease control in oligometastatic prostate cancer, analyze the evidence supporting cytoreductive prostatectomy, and review selected relevant ongoing clinical trials.
Definition and Diagnosis of Oligometastatic Prostate Cancer
Metastatic cancer is synonymous with advanced-stage disease, where malignant cells are disseminated through the systemic circulation. Cancers are, and this dissemination is, believed to occur in a stepwise fashion. In their 1995 article, Hellman and Weichselbaum postulated an oligometastatic state with limited metastasis burden as an intermediate state in the malignancy spectrum prior to widespread metastases.[37] In their opinion, oligometastatic disease may reflect a time point in the malignant process when local therapies can potentially achieve a durable response to treatment-or even cure.[37,38]
The rise in the diagnosis of oligometastatic prostate cancer is likely multifactorial, and may result from closer monitoring of patients, improved diagnosis of low-burden disease due to advances in imaging techniques, and improved survival secondary to the use of emerging new therapies.[39,40]
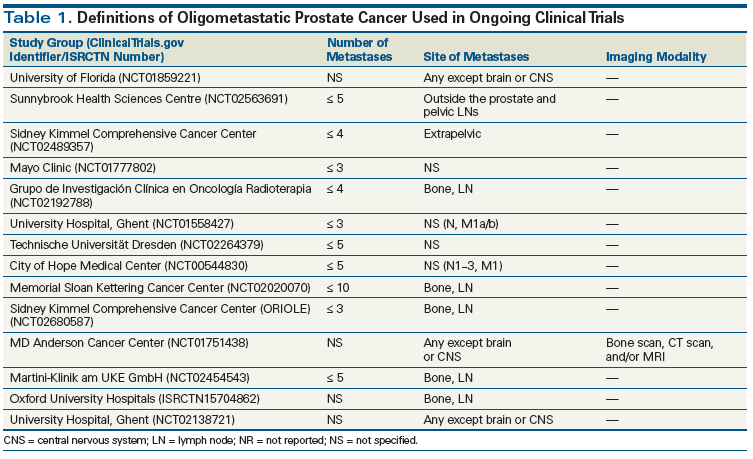
Currently, the European Association of Urology and the National Comprehensive Cancer Network suggest cross-sectional studies (such as CT scan or MRI) and 99mTc-methylene diphosphonate planar or single photon emission tomography bone scan as the standard of care to evaluate for presence of metastases to lymph nodes, bone, or viscera.[7-9] The number of metastases is the most commonly used criterion to distinguish between oligometastatic and widely metastatic disease. Some clinicians additionally emphasize the anatomic location of metastasis in determining the existence of an oligometastatic state. A review of the medical literature suggests wide variability in the criteria used to define oligometastatic prostate cancer, with different levels of evidence.[41] In most studies and ongoing trials, oligometastasis is defined as the presence of disease in five or fewer sites. Tables 1 and 2 summarize selected ongoing trials and previously published studies defining oligometastatic prostate cancer.
The Role of Novel Tracers
Regardless of the definition of oligometastases, if we believe there is a spectrum of disease behavior for metastatic prostate cancer, then the earlier we detect metastatic disease, when it has a lower burden, the more opportunity we have to treat either the primary malignancy or the oligometastases themselves. Conventional imaging with CT and bone scan lacks sensitivity and specificity for detecting small-volume or early metastases. Fortunately, we are in the midst of an imaging revolution in prostate cancer, with the emergence of newer imaging modalities that are better able to detect metastasis.
One such modality is positron emission tomography (PET). The novel PET radiotracers improve our ability to detect prostate cancer at lower levels of disease burden and hence may play a role in the diagnosis of oligometastatic prostate cancer. The most commonly used novel tracer in prostate cancer is 18F-sodium fluoride (18F Na), which has avidity for areas of bone undergoing remodeling, enabling detection of metastases. The improved spatial resolution offered by PET makes 18F Na PET/CT consistently superior to conventional bone scans in the detection of osseous metastases. However, despite improved sensitivity in diag nosing bone metastasis, 18F Na PET/CT relies on CT and/or MRI to evaluate for soft-tissue metastases.[42-44]
Other novel tracers that target the prostate cancers themselves include 11C-choline, 18F-fluoroethylcholine, 18F-FACBC, 18F-DCFPyL, and 68Ga-PSMA-11 (prostate-specific membrane antigen [PSMA], also known as glutamate carboxypeptidase). These tracers show advantages over use of the traditional PET tracer fluorodeoxyglucose, and over conventional CT and bone scan.[39,45,46]
PSMA-PET, in particular, shows promise in the detection of low-volume metastatic disease.[39,46-48] In a recent systematic review and meta-analysis by Perera et al, the 68Ga-PSMA-11 PET sensitivity and specificity for positive lesions confirmed by histopathology were both 86% in patients suspected of having disease recurrence.[49] On the other hand, van Leeuwen et al observed a relatively low detection rate of 54% for 68Ga-PSMA-11 PET/CT in a cohort of patients in whom conventional imaging was negative but who had suspected disease recurrence due to rising PSA (< 1.0 ng/mL) following RP.[46] In the majority of studies, novel imaging modalities demonstrate a high sensitivity and specificity for diagnosing metastatic prostate cancer in the setting of recurrent prostate cancer following initial radical treatment; however, these modalities are less reliable in the primary evaluation of patients with prostate cancer (who have not received local treatment).[39,46,48] This variability in the metastatic prostate cancer detection rate by PSMA-PET is affected by PSA, PSA doubling time (< 6 months), Gleason score, and clinical T stage, which have been shown to be associated with PSMA positivity.[39,48]
The Figure demonstrates the advantages of PSMA-MRI-PET imaging over conventional CT in identifying metastatic disease in a patient with prostate cancer and low metastatic burden.
While the aforementioned results are very promising, the current body of literature is not mature enough to support a role for newer-generation radiopharmaceuticals in the diagnosis of metastatic prostate cancer. The majority of published studies are based on retrospective series with small study populations and a heterogeneous cohort of men with prostate cancer. Accordingly, major urologic authorities-the American Urological Association and the European Association of Urology-consider conventional imaging modalities to be the diagnostic tools of choice for prostate cancer imaging. Comprehensive imaging trials should be considered to validate the methods and evaluate the sensitivity of PET/CT in metastatic prostate cancer, a technique that will also improve the ability to identify patients harboring oligometastatic prostate cancer (see Table 1).
Rationale for Local Therapy
Traditionally, metastatic prostate cancer has been considered to be an indication for systemic treatment in the form of ADT, regardless of the extent of metastatic disease.[9] Oligometastatic prostate cancer is understood to be a subset of metastatic prostate cancer but with a limited metastatic burden. Unlike diffuse systemic disease, however, established oligometastatic prostate cancer is unique in that a combination of primary tumor treatment, metastasis-directed therapy, and systemic therapy (ADT and chemotherapy) can potentially delay disease progression or in some cases be curative.[38,49]
Although no unifying strategy is established for the management of oligometastatic prostate cancer, there are conceptual ideas, preclinical studies, whole-genome sequencing results, and translational approaches that support the hypothesis that treatment of the primary tumor may provide benefit.
Metastasis is the most poorly understood aspect of carcinogenesis. This process requires regulatory genes and invasive factors that are crucial for the spreading of tumor cells into the circulation and for their lodging at distant sites.[50] The primary tumor, which has the propensity for constantly delivering these cells into circulation, can also prepare the distant premetastatic niche for successful implantation of disseminated cells.[51-54] Despite the administration of systemic therapy, tumor cells with fatal molecular features can survive in the primary tumor. Additionally, the primary tumor seems to be responsible for expression of signaling pathways that activate the epithelial-mesenchymal transition, which facilitates the release of tumor cells into systemic circulation and initiates the metastatic process.[30,55] Furthermore, by producing the necessary chemokines, angiogenic factors, fibronectin, bone marrow infiltrate cells, and other hormonal factors, the primary tumor appears to play a role in the development and maintenance of metastases.[56,57] Notably, the new metastatic foci can also act as a source for additional metastases while maintaining communication with the primary tumor by releasing tumor cells into systemic circulation.[57,58]
Genome sequencing technology has enabled us to thoroughly study tissue from both primary and metastatic prostate cancers.[59,60] These studies aid in the identification of founder mutations and processes that are relevant to metastatic progression, improve our understanding of the timing of metastasis, and establish relationships between primary and metastatic neoplasms and organ-specific signatures for metastatic subclonal branches within phylogenetic trees.[58,60,61] Hence, it is reasonable to hypothesize that interruption of this process by treatment of the primary tumor may alter the natural cancer biology and thereby limit disease progression.
To evaluate the role of cytoreductive treatment in metastatic prostate cancer, animal models have been developed. In a mouse model of metastatic prostate cancer, excision of the primary tumor (with or without systemic chemotherapy) resulted in a significant reduction in the size and number of metastases and prolonged survival.[62-64]
It has been hypothesized that oligometastatic prostate cancer has a less aggressive tumor biology and hence a longer natural history compared with widely metastatic disease. In fact, investigators have noted the effect of low-volume metastatic disease on survival. For example, Schweizer et al retrospectively reviewed 450 patients with biochemical recurrence following RP. Cox regression analysis demonstrated that patients with three or fewer metastatic foci had superior OS compared with those who had more than three metastases, although all patients had received treatment with ADT upon the development of metastases.[65]
While primary tumor debulking is an established concept in the management of a number of metastatic malignancies (such as renal cell carcinoma), the impact of cytoreductive surgery has only recently been evaluated in metastatic prostate cancer.[24] Nevertheless, studies reviewing the data on metastatic prostate cancer indicate that this approach may have merit. The exact mechanism by which decreasing the patient’s tumor burden improves survival is not well established, but there are several biologically and clinically plausible explanations.
Proponents of treating the primary tumor in the setting of oligometastatic prostate cancer argue that local treatment offers potential benefits that include not only decreased tumor burden, but also decreased disease-related morbidity and need for palliative intervention, increased time to development of castration-resistant prostate cancer (CRPC), and improved PFS and cancer-specific survival (CSS) outcomes.[65-68]
Won et al evaluated the role of local primary tumor treatment (with RP and RT) vs no treatment in men who developed CRPC.[68] In this study, treatment of the primary tumor (either via RP or RT) significantly reduced the incidence of subsequent local complications compared with patients who had no such treatment. Interestingly, RP was associated with a significantly lower probability of local complications compared with RT.
Ghavamian et al, in a retrospective matched-pair analysis of 79 men with lymph node–positive prostate cancer, compared patients who underwent RP, lymph node dissection, and orchiectomy with patients who had undergone orchiectomy and lymph node dissection without treatment of the primary tumor. The RP cohort had a significantly improved 10-year OS rate (66% vs 28%; P < .001) and CSS rate (79% vs 39%; P < .001).[69] In a study of 158 patients with lymph node–positive prostate cancer, 108 patients received RP in addition to lymphadenectomy and early ADT. In a matched-pair analysis, the 50 patients who did not receive RP demonstrated worse clinical PFS and CSS, although the authors point out the likelihood of selection bias favoring RP in patients with a lower burden of disease.[70] Similarly, results of the Eastern Cooperative Oncology Group (ECOG) trial 3886 (RP followed by early ADT vs delayed ADT for pN-positive men) demonstrate improved oncologic outcomes compared with those of the European Organisation for Research and Treatment of Cancer trial 30846 (which compared early vs delayed ADT in pN1-3M0 disease, with no treatment of the primary tumor). Considering the main difference between the two trials, namely receipt of RP in ECOG 3886, it appears that the efficacy of ADT was improved in the patients who underwent RP.[71,72] One important point is that these studies did not distinguish whether the metastatic disease was oligometastatic or polymetastatic; thus, it is impossible to ascertain the magnitude of the favorable association between RP and outcome for this specific subgroup.
Regarding patients with bone metastases, several studies have reported on the survival benefit of local surgery, due to the resultant better response to systemic therapies. The post-hoc analysis of Southwest Oncology Group trial 8894, which randomized 1,286 men with metastatic prostate cancer to orchiectomy and placebo vs orchiectomy and flutamide, showed that prior RP improved CSS.[31] More recently, a subgroup analysis of the IMPACT study (which evaluated sipuleucel-T in men with CRPC) showed better results in the cohort of patients who had undergone prior RP.[73] The caveat is that these post-hoc analyses are highly prone to selection bias.
Population-Based Data on Outcomes of Local Therapy in Metastatic Prostate Cancer
There is a growing body of evidence to support the role of local therapy in the setting of metastatic prostate cancer. Culp et al used Surveillance, Epidemiology, and End Results (SEER) program data from 2004 through 2010 to assess the impact of local treatment (with RP or brachytherapy) on OS and CSS in men with metastatic prostate cancer.[74] Patients who received local therapy were younger, more likely to have Gleason scores of 7 or lower, and more likely to have clinical stage N0 disease. After multivariate adjustment and competing-risk analysis, patients who received local therapy were shown to have a significantly improved cancer-specific mortality (CSM) compared with those who did not receive local therapy. In the locally treated patients, the 5-year disease-specific survival rates were 75.8% for those who underwent RP and 61.3% for patients treated with brachytherapy; in contrast, the rate was 48.7% for patients who received no local treatment. Importantly, higher rates of CSM were associated with patient age of 70 years and older, cT4 disease, PSA levels of 20 ng/mL and above, high-grade disease, and pelvic lymphadenopathy. In another study using SEER data, Antwi et al performed a propensity score analysis to account for baseline differences between metastatic prostate cancer patients who received local therapy and those who did not.[75] Local therapy was associated with improved CSM, and RP in particular was associated with a 72% improvement in CSM (hazard ratio, 0.28; 95% CI, 0.20–0.39).
However, these two studies have limitations: despite the attempts to control for differences between the subgroups treated with local therapy, there may be residual unmeasured confounding bias from unknown factors, and fewer than 5% of the study population received local therapy. Therefore, the point estimates showing benefit are likely not generalizable to all patients with metastatic disease.
Fossati et al investigated the role of primary tumor treatment on CSM in patients with metastatic prostate cancer using SEER database information from 2004 through 2011. They demonstrated that the benefit of local treatment was directly tied to the risk of CSM. Patient age and tumor-related characteristics (PSA level at diagnosis, Gleason score, TNM stage) were used to calculate the risk of CSM, which was plotted against CSM-free survival at 3 years after diagnosis. Local treatment of the primary tumor improved the CSM-free survival rate only in patients who had a lower risk of CSM.[76]
More recently, Leyh-Bannurah et al evaluated the role of local therapy vs no local therapy in metastatic prostate cancer using SEER data from 2004 through 2013.[77] Of 13,692 patients with metastatic prostate cancer, just 474 (3.4%) were treated with RP or RT. Multivariable competing-risk regression analyses after propensity score matching demonstrated lower CSM in the local therapy cohort (subhazard ratio, 0.40; 95% CI, 0.32–0.50). Additionally, patients who underwent RP demonstrated a lower CSM compared with the RT cohort (subhazard ratio, 0.59; 95% CI, 0.35–0.99). The lowest CSM was observed in patients with Gleason score 7, clinical stage T3, and M1a disease; these findings underscore the importance of disease risk stratification when selecting patients for local therapy. Limitations of the study that warrant consideration include its retrospective design, use of population-based data, and the small percentage of metastatic prostate cancer patients who received local therapy.
KEY POINTS
- The traditional approach to metastatic prostate cancer (systemic hormonal therapy) was challenged by the findings of the CHAARTED, STAMPEDE, GETUG-AFU 15, and LATITUDE trials.
- Diagnostic modalities using novel radiotracers (ie, PSMA-PET) proved to be detrimental in identifying patients with low metastatic burden and otherwise negative conventional imaging (ie, CT and bone scan) results.
- Studies suggest a paradigm shift toward an aggressive multimodal approach in oligometastatic prostate cancer, with treatment of primary tumor considered in men < 70 years old, clinical stage < T4, prostate-specific antigen (PSA) level < 20 ng/ mL, Gleason score < 8, < 35 bone metastases and no bulky lymphadenopathy, PSA nadir <1 ng/mL, and no disease progression while on hormonal therapy for 6 months.
Single-Center Data on Outcomes of Local Therapy in Metastatic Prostate Cancer
Heidenreich et al were the first to report the results of a case-control study examining the role of RP in a cohort of men with metastatic prostate cancer.[78] Twenty-three men with metastatic prostate cancer (three or fewer lesions on bone scan; no visceral or extended lymph node metastases; and response to ADT, with a PSA nadir < 1.0 ng/mL after 6 months of ADT) were compared with a cohort of men (n = 38) with similar clinical and pathologic characteristics. Outcomes of interest were castration resistance, clinical progression, and survival. The RP cohort demonstrated significantly increased time to castration resistance (median, 40 months vs 29 months; P = .014), freedom from clinical progression (median, 38.6 months vs 26.5 months; P = .032), and improved OS rate (91.3% vs 78.9%; P = .048).
Gandaglia et al assessed perioperative and long-term oncologic outcomes of RP in a selected cohort of patients with oligometastatic disease. In this highly selected cohort, RP was found to be safe and associated with a relatively low rate of morbidity. The 7-year clinical PFS and CSM-free survival rates were 45% and 82%, respectively.[79] In another retrospective study, Sooriakumaran et al examined the perioperative and short-term complications after RP in patients with metastatic prostate cancer. While RP was found to be technically feasible and safe in men with metastatic prostate cancer, it was associated with a high rate of positive margins (57%), an advanced tumor stage (78% stage T3 and higher), and a high rate of positive nodes (72%). As expected, patients with bone metastasis (M1b) had worse OS and CSS than those with nodal disease (M1a).[80]
Based on the published literature, not all patients with metastatic prostate cancer appear to be suitable candidates for local therapy; collectively, an appropriate patient for primary tumor treatment is one younger than 70 years of age, with clinical stage less than T4, PSA level below 20 ng/mL, no high-grade disease (Gleason score ≥ 8), and a limited metastases burden (no more than three to five bone metastases and no bulky lymphadenopathy) who does not experience disease progression during a 6-month course of ADT and has a PSA nadir of less than 1 ng/mL.[74,78]
Future Directions
The current literature provides only a limited picture of the role of local therapy in oligometastatic prostate cancer. Institutional studies are retrospective in nature and are reporting outcomes observed in small numbers of patients during a short follow-up period. The large population-based studies also have weaknesses, since they do not provide data on crucial parameters known to be associated with survival (eg, comorbidities, number and sites of metastases, and use of ADT or chemotherapy) and are prone to selection bias.
Despite their limitations, the results of published studies are hypothesis-generating and should be used to design clinical trials to determine the role of local surgery in metastatic prostate cancer and to select patients who are more likely to benefit from surgery.
To define the role of surgical treatment of the primary tumor in metastatic prostate cancer, as well as the impact of systemic therapy and RT, several trials are now enrolling patients (Table 1). Current trials in oligometastatic prostate cancer limit the maximum number of metastatic lesions to five bone and/or visceral metastases. (The exception is the ongoing trial at Memorial Sloan Kettering Cancer Center [ClinicalTrials.gov identifier: NCT02020070], which enrolls patients with up to 10 lesions.) The majority of these trials are in the recruiting phase, and the short-term results are expected in the next 3 to 4 years. Considering the scope of the ongoing trials, it is safe to predict that a combination of primary tumor treatment (surgery and/or radiation), metastasis-directed therapy (surgery and/or radiation), and systemic therapy (hormonal and chemotherapy) will delay disease progression or even provide the possibility of cure to patients with oligometastatic prostate cancer.
Conclusion
Oligometastatic prostate cancer is considered a disease state with a limited metastatic burden (five or fewer lesions). Given their improved sensitivity and specificity compared with traditional imaging, novel imaging modalities offer the ability to identify patients with “true” oligometastatic disease. The current literature suggests that a paradigm shift is underway in the management of oligometastatic prostate cancer. A more aggressive and multimodal approach (using systemic therapy, local therapy, and metastasis-directed therapies) in select groups of patients may offer opportunities to eradicate malignancy or delay its progression. However, the available data are insufficient to draw firm conclusions about the effectiveness of aggressive therapies on OS or CSS. Prospective well-controlled trials assessing the role of treatment of the primary tumor in the metastatic setting are well underway, and the results will be available in the near future.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Acknowledgment:Ricardo Leão is supported by the Foundation for Science and Technology, Government of Portugal, SFRH/BD/102232/2014 Individual Doctoral Grant.
References:
1. Ryan CJ, Elkin EP, Small EJ, et al. Reduced incidence of bony metastasis at initial prostate cancer diagnosis: data from CaPSURE. Urol Oncol. 2006;24:396-402.
2. Banerji JS, Wolff EM, Massman JD 3rd, et al. Prostate needle biopsy outcomes in the era of the U.S. Preventive Services Task Force recommendation against prostate specific antigen based screening. J Urol. 2016;195:66-73.
3. Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol. 2015;194:1587-93.
4. Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013). Prostate Cancer Prostatic Dis. 2016;19:395-7.
5. Mathieu R, Korn SM, Bensalah K, et al. Cytoreductive radical prostatectomy in metastatic prostate cancer: does it really make sense? World J Urol. 2017;35:567-77.
6. Crawford ED, Higano CS, Shore ND, et al. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. 2015;194:1537-47.
7. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124-37.
8. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467-79.
9. Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer. Version 1.2016. J Natl Compr Canc Netw. 2016;14:19-30.
10. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737-46.
11. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-77.
12. Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149-58.
13. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-60.
14. Wu JN, Fish KM, Evans CP, et al. No improvement noted in overall or cause-specific survival for men presenting with metastatic prostate cancer over a 20-year period. Cancer. 2014;120:818-23.
15. James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67:1028-38.
16. Gandaglia G, Sun M, Trinh QD, et al. Survival benefit of definitive therapy in patients with clinically advanced prostate cancer: estimations of the number needed to treat based on competing-risks analysis. BJU Int. 2014;114:E62-E69.
17. Bastian PJ, Boorjian SA, Bossi A, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61:1096-106.
18. Touijer KA, Mazzola CR, Sjoberg DD, et al. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur Urol. 2014;65:20-5.
19. Bekelman JE, Mitra N, Handorf EA, et al. Effectiveness of androgen-deprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015;33:716-22.
20. Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104-11.
21. Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301-8.
22. Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930-42.
23. Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248-59.
24. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655-9.
25. Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219-28.
26. Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966-70.
27. Nitta T, Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer. 1995;75:2727-31.
28. Temple LK, Hsieh L, Wong WD, et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475-84.
29. Resel Folkersma L, San José Manso L, Galante Romo I, et al. Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology. 2012;80:1328-32.
30. Tzelepi V, Efstathiou E, Wen S, et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. J Clin Oncol. 2011;29:2574-81.
31. Thompson IM, Tangen C, Basler J, Crawford ED. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002;168:1008-12.
32. Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956-62.
33. James ND, Spears MR, Clarke NW, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016;2:348-57.
34. Lin CC, Gray PJ, Jemal A, Efstathiou JA. Androgen deprivation with or without radiation therapy for clinically node-positive prostate cancer. J Natl Cancer Inst. 2015;107(7).
35. Rusthoven CG, Carlson JA, Waxweiler TV, et al. The impact of definitive local therapy for lymph node-positive prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2014;88:1064-73.
36. Tward JD, Kokeny KE, Shrieve DC. Radiation therapy for clinically node-positive prostate adenocarcinoma is correlated with improved overall and prostate cancer-specific survival. Pract Radiat Oncol. 2013;3:234-40.
37. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10.
38. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378-82.
39. Evangelista L, Briganti A, Fanti S, et al. New clinical indications for (18)F/(11)C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70:161-75.
40. Khoo V. Is there another bite of the cherry? The case for radical local therapy for oligometastatic disease in prostate cancer. Eur Urol. 2016;69:13-4.
41. Tosoian JJ, Gorin MA, Ross AE, et al. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14:15-25.
42. Apolo AB, Lindenberg L, Shih JH, et al. Prospective study evaluating Na18F PET/CT in predicting clinical outcomes and survival in advanced prostate cancer. J Nucl Med. 2016;57:886-92.
43. Poulsen MH, Petersen H, Hoilund-Carlsen PF, et al. Spine metastases in prostate cancer: comparison of technetium-99m-MDP whole-body bone scintigraphy, [(18) F]choline positron emission tomography(PET)/computed tomography (CT) and [(18) F]NaF PET/CT. BJU Int. 2014;114:818-23.
44. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-9.
45. Cho SY, Szabo Z. Molecular imaging of urogenital diseases. Semin Nucl Med. 2014;44:93-109.
46. van Leeuwen PJ, Stricker P, Hruby G, et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732-9.
47. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226-35.
48. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926-37.
49. O’Shaughnessy MJ, McBride SM, Vargas HA, et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early-stage metastatic prostate cancer. Urology. 2017;102:164-72.
50. Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529-37.
51. Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315-26.
52. Miyamoto DT, Sequist LV, Lee RJ. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat Rev Clin Oncol. 2014;11:401-12.
53. Nguyen DX. Tracing the origins of metastasis. J Pathol. 2011;223:195-204.
54. Sceneay J, Smyth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449-64.
55. Taplin ME, Montgomery B, Logothetis CJ, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32:3705-15.
56. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679-95.
57. Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089-93.
58. Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109-13.
59. Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353-7.
60. Hong MK, Macintyre G, Wedge DC, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun. 2015;6:6605.
61. Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114-7.
62. Cifuentes FF, Valenzuela RH, Contreras HR, Castellon EA. Development of an orthotopic model of human metastatic prostate cancer in the NOD-SCIDgamma mouse (Mus musculus) anterior prostate. Oncol Lett. 2015;10:2142-8.
63. Cifuentes FF, Valenzuela RH, Contreras HR, Castellon EA. Surgical cytoreduction of the primary tumor reduces metastatic progression in a mouse model of prostate cancer. Oncol Rep. 2015;34:2837-44.
64. Kadmon D, Heston WD, Fair WR. Treatment of a metastatic prostate derived tumor with surgery and chemotherapy. J Urol. 1982;127:1238-42.
65. Schweizer MT, Zhou XC, Wang H, et al. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol. 2013;24:2881-6.
66. Ost P, Decaestecker K, Lambert B, et al. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate. 2014;74:297-305.
67. Wiegand LR, Hernandez M, Pisters LL, Spiess PE. Surgical management of lymph-node-positive prostate cancer: improves symptomatic control. BJU Int. 2011;107:1238-42.
68. Won AC, Gurney H, Marx G, et al. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013;112:E250-E255.
69. Ghavamian R, Bergstralh EJ, Blute M, et al. Radical retropubic prostatectomy plus orchiectomy versus orchiectomy alone for pTxN+ prostate cancer: a matched comparison. J Urol. 1999;161:1223-7.
70. Steuber T, Budaus L, Walz J, et al. Radical prostatectomy improves progression-free and cancer-specific survival in men with lymph node positive prostate cancer in the prostate-specific antigen era: a confirmatory study. BJU Int. 2011;107:1755-61.
71. Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472-9.
72. Schroder FH, Kurth KH, Fossa SD, et al. Early versus delayed endocrine treatment of pN1-3 M0 prostate cancer without local treatment of the primary tumor: results of European Organisation for the Research and Treatment of Cancer 30846-a phase III study. J Urol. 2004;172:923-7.
73. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
74. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058-66.
75. Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: a population-based, propensity score analysis. Cancer Epidemiol. 2014;38:435-41.
76. Fossati N, Trinh QD, Sammon J, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol. 2015;67:3-6.
77. Leyh-Bannurah SR, Gazdovich S, Budaus L, et al. Local therapy improves survival in metastatic prostate cancer. Eur Urol. 2017;72:118-24.
78. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832-8.
79. Gandaglia G, Fossati N, Stabile A, et al. Radical prostatectomy in men with oligometastatic prostate cancer: results of a single-institution series with long-term follow-up. Eur Urol. 2017;72:289-92.
80. Sooriakumaran P, Karnes J, Stief C, et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur Urol. 2016;69:788-94.
81. Tabata K, Niibe Y, Satoh T, et al. Radiotherapy for oligometastases and oligo-recurrence of bone in prostate cancer. Pulm Med. 2012;2012:541656.
82. Ahmed KA, Barney BM, Davis BJ, et al. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2013;2:215.
83. Berkovic P, De Meerleer G, Delrue L, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11:27-32.
84. Schick U, Jorcano S, Nouet P, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013;52:1622-8.
85. Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014;9:135.