Reassessing Adjuvant Treatment of Early-Stage Breast Cancer in Elderly Women: Focus on Findings from 2007 ASCO Meeting
Approximately 212,920 new cases of invasive breast cancer were estimated to occur in the United States in 2006.1 The incidence rate has continued to rise slowly over the past 20 years due to the continued increase of breast cancer in women aged 50 and older (375 cases per 100,000 women), peaking at 75 to 79 years of age (525 per 100,000 women).
Continuing Medical Education InformationReassessing Adjuvant Treatment of Early-Stage Breast Cancer in Elderly Women: Focus on Findings from 2007 ASCO Meeting
Activity Release Date: September 15, 2007
Activity Expiration Date: September 15, 2008
About the Activity
This activity is based on a brief article developed as part of the E-Update Series and posted on the Web. The series is geared to oncologists and addresses new treatments of cancer or modifications thereof.
This activity has been developed and approved under the direction of Beam Institute.
Activity Learning Objectives
After reading this article, participants should be able to:
(a) Discuss the current role of radiation therapy after breast-conserving surgery in older women with early breast cancer.
(b) Compare the effectiveness and side-effect profiles of tamoxifen versus aromatase inhibitors such as letrozole and anastrozole in the treatment of breast cancer.
(c) Identify the preferred adjuvant hormonal agents for elderly women with early breast cancer.
(d) Appreciate the connection between aromatase inhibitors and bone health as well as the importance of screening older women for risk factors for osteoporosis.
Target Audience
This activity targets physicians in the fields of oncology and hematology.
Accreditation
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Beam Institute and The Oncology Group. Beam Institute is accredited by the ACCME to provide continuing medical education for physicians.
Continuing Education CreditAMA PRA Category 1 Credit™
The Beam Institute designates this educational activity for a maximum of 2 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Compliance Statement
This activity is an independent educational activity under the direction of Beam Institute. The activity was planned and implemented in accordance with the Essential Areas and policies of the ACCME, the Ethical Opinions/Guidelines of the AMA, the FDA, the OIG, and the PhRMA Code on Interactions with Healthcare Professionals, thus assuring the highest degree of independence, fair balance, scientific rigor, and objectivity.
However, Beam Institute, the Grantor, and CMPMedica shall in no way be liable for the currency of information or for any errors, omissions, or inaccuracies in the activity. Discussions concerning drugs, dosages, and procedures may reflect the clinical experience of the author(s) or may be derived from the professional literature or other sources and may suggest uses that are investigational in nature and not approved labeling or indications. Activity participants are encouraged to refer to primary references or full prescribing information resources. The opinions and recommendations presented herein are those of the author(s) and do not necessarily reflect the views of the provider or producer.
Financial Disclosures
Drs. Lichtman and Seo have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in the article. Dr. Kimmick serves on the speakers' bureau for AstraZeneca and Pfizer; and receives research support from AstraZeneca.
Copyright
Copyrights owned by Beam Institute, a division of CME LLC. Copyright 2007, CME LLC. All rights reserved.
Contact Information
We would like to hear your comments regarding this or other activities provided by Beam Institute. In addition, suggestions for future activities are welcome. Contact us at:
Address:
Director of Continuing Education
Beam Institute
11 West 19th Street, 3rd Floor
New York, NY 10011-4280
Phone: 888-618-5781
Fax: 212-600-3050
e-mail:beaminstitute@cmp.com
Introduction
Approximately 212,920 new cases of invasive breast cancer were estimated to occur in the United States in 2006.1 The incidence rate has continued to rise slowly over the past 20 years due to the continued increase of breast cancer in women aged 50 and older (375 cases per 100,000 women), peaking at 75 to 79 years of age (525 per 100,000 women).1 If these incidence rates remain stable, by 2030, two-thirds of patients with breast cancer will be 65 years of age or older, and by 2050, the total number of breast cancer cases will double due to the aging of the population.2
Less aggressive management of breast cancer in older women has been widely reported.3-5 Older women may be more likely to have nonstandard initial therapy than younger women4 and appear to be less likely to be represented in clinical trials.6,7
Although not unique to older individuals, chronic disease is more common with advancing age. Almost 80% of people older than age 65 have at least one chronic disease, and approximately one-third have three or more chronic diseases.8 This finding is important for two reasons: First, the choice of therapy is influenced by the presence of coexisting diseases that may increase complications; and, second, in clinical trials, the best measure of success of cancer treatment is overall survival, which may be limited by coexisting illness, making the benefit of therapy difficult to evaluate.
The guiding principles of breast cancer management are early detection, aggressive local therapy to prevent recurrence of breast cancer at the primary site, and systemic therapy to prolong survival by preventing the development or slowing the progression of metastatic disease. These principles apply to treatment of older women but are more complicated because of the presence of other illnesses that may also limit survival. In some cases, the focus of treatment is then redirected to improving the remaining years, either by maintaining breast cancer recurrence-free survival and/or quality of life.
The results of studies focusing on older women with breast cancer are heterogeneous because of the varying definitions of “elderly.” Some studies define “elderly” as any postmenopausal woman, whereas others use the cutoff age of 65. However, with improved life expectancy in the United States, age 65 should be considered a younger-old person. In this article, we initiate the discussion of adjuvant radiotherapy and hormonal therapy by looking at two abstracts from the 2007 American Society of Clinical Oncology (ASCO) meeting and use their findings as a springboard to evaluate the published literature, specifically focusing on women aged 75 or older with early-stage breast cancer.
The Role of Radiation Therapy After Breast-Conserving Surgery in Older Women
Abstract from 2007 ASCO Meeting
At the 2007 ASCO meeting, Swenson and colleagues used Surveillance Epidemiology and End Results (SEER) registry data to explore the utility of radiation therapy after breast-conserving surgery (BCS) in women aged 65 and older with stages I and II breast cancer diagnosed between 1998 and 2002.9 In their retrospective analysis, which included 28,808 women, they found 5-year relative survival was not significantly improved by radiation therapy after BCS for patients with stage I tumors who were between the ages of 85 and 94.
Of the 20,400 women with stage I disease, 72% had received radiation therapy; of the 8,408 women with stage II disease, 66% had received adjuvant radiotherapy. For stage II disease, the overall 5-year relative survival rate was 0.99 (95% confidence interval [CI], 0.98-1.02) with radiotherapy and 0.83 (95% CI, 0.79-0.87) without radiotherapy, indicative of the need for radiation therapy after BCS in older women with node-positive disease. For stage I disease, the overall 5-year Kaplan-Meier relative survival was 1.00 (95% CI, 0.99-1.01) compared with 0.96 (95% CI, 0.93-0.99) without radiotherapy, suggesting that the benefit of radiation therapy after BCS in stage I breast cancer is less important. Table 1 presents the investigators’ results by age. In the 75-84 year age group, twice as many women received radiotherapy as not, whereas in the 85-94 year age group, less than half received radiotherapy.
Click to enlarge
Although these results are intriguing because of the large numbers of subjects in their 80s and 90s represented, the study carries the usual caveats of any retrospective analysis. Was there a bias in which patients received radiotherapy? For instance, were the women with more comorbidities the women who did not receive radiotherapy? Answers to the following questions are needed. What was the absolute number of death events? What were the CIs in the age-based subgroup analysis? Were the deaths related to the disease? Was adjuvant hormonal therapy used uniformly? What was the rate of disease-free survival?
This report adds to the literature by providing another area to explore in future prospective trials. One other retrospective registry study, with 7.5 years of median follow-up, showed an overall survival advantage with adjuvant radiotherapy in older women.10 Based on these reports, delivery of radiation therapy after BCS remains the preferred management strategy, regardless of age.
Studies Focusing on CALGB C9343 Trial
The seminal randomized trials have shown that radiation therapy after BCS decreases the rate of breast cancer recurrence without improving overall survival.11-13 For women aged 70 and older only, the Cancer and Leukemia Group B (CALGB) conducted a randomized, controlled trial (CALGB C9343) of 636 women with stage I (tumors up to 2 cm and clinically negative axillary nodes) estrogen receptor-positive breast cancer. Women were randomized to receive lumpectomy, radiation therapy, and tamoxifen or lumpectomy and tamoxifen alone.14 About 55% of women in each arm were 75 years or older.
With a median follow-up of 5 years, there was no difference in distant metastases or 5-year overall survival between the groups. The only significant finding was a difference in the local or regional recurrence rate (radiation therapy, 1%; no radiation therapy, 4%). Quality-of-life questions of cosmesis and adverse events favored the tamoxifen-alone group. The 5-year results of this prospective trial justify the omission of radiation therapy in women older than age 75 who were to be treated with tamoxifen for a T1, hormone receptor-positive breast cancer.
To determine whether these findings held true in the community, Smith and colleagues performed a retrospective study by selecting women who met the eligibility criteria of the CALGB C9343 study from linked SEER–Medicare databases from 1992 to 1999.15 A total of 8,724 women were identified; the median age was 77 years (range, 73 to 82 years), and 73% of the women had received radiation therapy.
At 5 years, the absolute benefit of adjuvant radiation therapy remained low but significant for both recurrence (2%), which was similar to the CALGB C9343 trial, and for subsequent mastectomy (4%), which was not significant in the CALGB C9343 trial. These risk reductions increased when patients were evaluated at 8-year follow-up; thus, women who lived longer were more likely to benefit from radiation therapy (Table 2). Therefore, for older women whose life expectancy is more than 5 years, radiation therapy should be offered after BCS.
Click to enlarge
Taking their study one step farther, Smith and colleagues used Medicare data to calculate a Charlson comorbidity index for each subject, which resulted in 55% and 69% of women having no comorbid illness in the no-radiation and radiation arms, respectively. An adjusted number needed to treat (NNT) was described to indicate the number of patients who would require adjuvant radiotherapy to prevent one local recurrence, controlled for 8-year age- and comorbidity-adjusted life expectancy (Table 3).15,16
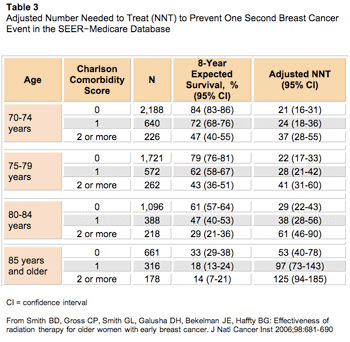
Click to enlarge
In this group of older women with T1, hormone receptor-positive breast cancer, the following groups had an adjusted NNT within the accepted bounds of other preventive treatment recommendations (ie, 21 women require 10 years of antihypertensive treatment to prevent one coronary artery disease event) to justify the use of adjuvant radiation therapy after BCS: age 70-74 with no or one comorbidity, age 75-79 with no or one comorbidity, and age 80- 84 without comorbid illness.15,17
PRIME Trial
Additional information in this area will come from the Postoperative Radiotherapy In Minimum Risk Elderly (PRIME) trial. In this study, 240 women aged 65 years or older with T0-2 node-negative breast cancer are to be randomized to receive radiation therapy and endocrine therapy or endocrine therapy alone after BCS. Study endpoints include recurrence, morbidity/cosmesis, quality of life, and cost-effectiveness. This trial has completed accrual, and a report of results is scheduled for October 2007.18
In summary, lumpectomy followed by adjuvant hormonal therapy, without radiation therapy, is an option in women older than 75 years with hormone receptor-positive, T1N0 stage breast cancer and more than one comorbid illness. However, for healthy 75 to 84-year-olds without comorbid illness, or 75 to 79-year-olds with only one comorbidity, adjuvant radiotherapy should be thoughtfully considered.
Comparison of Adjuvant Hormonal Therapies
Tamoxifen Alone
A great deal is known about tamoxifen through decades of its use. The Early Breast Cancer Trialists’ Collaborative Group overview of randomized trials showed that 5 years of tamoxifen reduced the annual recurrence and breast cancer mortality rates by 39% and 31%, respectively.19 This benefit held true in women older than age 70. The protective effect of tamoxifen continued past the duration of active treatment, up to 5 years post treatment for breast cancer recurrence, and beyond 5 years for breast cancer mortality. Interestingly, even 1-2 years of tamoxifen therapy confers some benefit, with a reduction in the annual recurrence and breast cancer mortality rates of 21% and 15%, respectively.
On the other hand, the toxicities from tamoxifen are well known; they include cerebrovascular events, thromboembolic events, endometrial carcinoma and vasomotor symptoms. The risk of these adverse events increases with age (Table 4).20,21
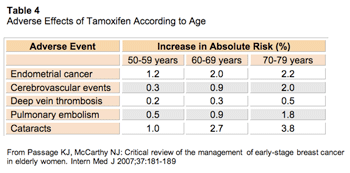
Click to enlarge
Aromatase Inhibitors vs Tamoxifen
Aromatase inhibitors (AIs) provide further reductions in breast cancer recurrence when compared with tamoxifen in the adjuvant setting.22-26 A survival benefit was also seen in patients with node-positive disease in the extended letrozole (Femara) arm of the NCIC−CTG (National Cancer Institute of Canada Clinical Trials Group) MA.17 trial.27 Current questions remain unanswered regarding the sequence, duration, and choice of AIs. Because AIs have a different toxicity spectrum than tamoxifen, oncologists choose adjuvant hormonal therapy by toxicity risk for an individual elderly woman. However, as the trials mature and gain longer follow-up, reports indicate that individual AIs have different toxicity profiles.
At the 2007 ASCO meeting, Crivellari et al reported on an age-based subgroup analysis of efficacy and safety among women in the Breast International Group (BIG) 1-98 trial from the continuous adjuvant tamoxifen and letrozole for 5 years arms.28 The 4,922 participants were divided into the following age groups: women younger than 65 years old, those 65 to 74 years old, and those 75 years old and older. With a median follow-up of 40 months and use of subpopulation treatment effect pattern plot analyses, the investigators demonstrated that regardless of age, letrozole provided a greater reduction in recurrence risk than did tamoxifen. However, women aged 75 and older were the least likely group to complete trial treatment, equally in both the letrozole and tamoxifen arms.
Regarding adverse events, toxicities differed when viewed by age, with greater bone and any targeted event (cardiac, vascular, nausea, vomiting, and vaginal bleeding; grade 3 or higher) in the women aged 75 and older who were treated with letrozole. Interestingly, there was no difference in thromboembolic and cardiac adverse events (grade 3 or higher) in this age group (Table 5). Although there are a relatively small number of women older than age 75, and full description of the absolute numbers and analysis need to be presented, overall these results continue to highlight differences in adverse events between letrozole and anastrozole (Arimidex).
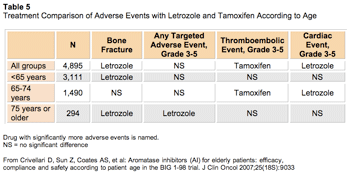
Click to enlarge
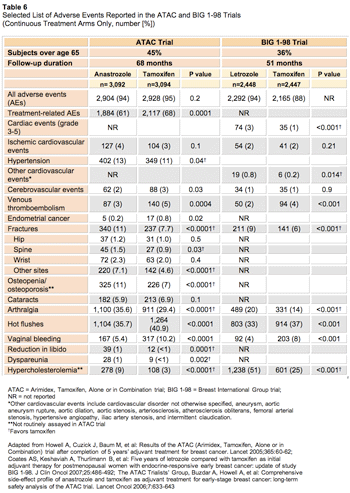
Of the AIs, more is known about anastrozole, because the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial has the longest follow-up at 68 months, with 92% of subjects completing assigned randomized therapy.22 In the ATAC trial, 45% of subjects (4,229 of 9,366 women) were 65 years or older.29 Thus, approximately 2,866 women over the age of 65 were in the two single-drug arms (tamoxifen alone and anastrozole alone).
Anastrozole significantly prolonged disease-free survival (hazard ratio [HR], 0.87; 95% CI, 0.78-0.97; P = 0.01) and time to recurrence (HR, 0.79; 95% CI, 0.70-0.90; P = 0.0005), with no effect on overall survival at a median follow-up of 68 months (HR, 0.97; 95% CI, 0.85-1.12).22 As was seen in trials of adjuvant tamoxifen versus placebo, improved survival is expected with longer follow-up. The ATAC trialists refrain from describing subgroups, unless these subgroups show statistically significant differences compared with the overall effect through interaction or heterogeneity analyses.30
Click to enlarge
A comprehensive side-effect profile of anastrozole compared with that of tamoxifen has been described, and some of the findings are shown in Table 6.22,31 In the ATAC trial, there were equivalent numbers of reported adverse events in the anastrozole and tamoxifen groups (94% vs 95%; P = 0.2). As expected, anastrozole was associated with fewer endometrial cancers, thromboembolic and cerebrovascular events, and vasomotor and gynecologic symptoms; anastrozole was linked to an increase in vaginal dryness with associated sexual dysfunction, joint symptoms, and fractures compared with tamoxifen. The annual fracture rate remained constant (22.6 vs 15.6 fractures per 1,000 women years; HR, 1.43; 95% CI, 1.21-1.68; P < 0.0001). Bisphosphonate use did not confound these results, since their usage was low (10% in the anastrozole group and 7% in the tamoxifen group). Lipid concentrations were not routinely assessed, but documented hypercholesterolemia was higher in the anastrozole group (278 [9%] vs 108 [3%]; P < 0.0001). Adverse events leading to withdrawal of medication were less common in the anastrozole arm (6%) than in the tamoxifen arm (9%; P = 0.0005). These findings suggest that anastrozole may be a more effective and better-tolerated option than tamoxifen, with the benefit greatest at 1 to 2 years of treatment.31
The BIG 1-98 study is a four-arm trial of letrozole versus tamoxifen given continuously or sequentially. Results from the two continuous therapy arms have been separated from the larger study for analysis.23 These arms consisted of 4,922 women, of whom 36% (1,795) were aged 65 and older. With a median follow-up of 51 months and 43% of the women still on therapy, there was a disease-free survival benefit with letrozole compared with tamoxifen (HR, 0.82; 95% CI, 0.71-0.95; P = 0 .007) but no survival benefit (HR, 0.91; 95% CI, 0.75-1.11; P = 0.35). In subgroup analysis, older women over the age of 65 experienced similar benefits as the overall group.
More patients receiving letrozole reported at least one adverse event of any grade than patients receiving tamoxifen (93.6% vs 88.5%). As expected, patients on tamoxifen experienced more thromboembolic events, endometrial pathology, vasomotor symptoms, and vaginal bleeding, whereas women on letrozole had more bone fractures, arthralgia, elevated cholesterol levels, and other cardiovascular events (cardiovascular disorder not otherwise specified, aneurysm, aortic aneurysm rupture, aortic dilation, aortic stenosis, arteriosclerosis, atherosclerosis obliterans, femoral arterial stenosis, hypertensive angiopathy, iliac artery stenosis, and intermittent claudication). There was also a significant trend for higher-grade cardiac events in the letrozole group than in the tamoxifen group (Table 6).
The vast majority of patients complained of some adverse symptom in the adjuvant trials of AIs versus tamoxifen, with about two-thirds of symptoms probably related to treatment. For older breast cancer patients, the most concerning side effects involved osteoporosis and cardiovascular disease, diseases already common in this population. Overall, use of AIs resulted in fewer serious adverse events, except for fractures. Even though there was no increase in hip fractures in the ATAC trial, spinal fractures still convey significant morbidity in older women.32,33 With respect to cardiovascular disease, there is concern that AI therapy may be related to higher cholesterol levels. Elevated cholesterol levels were reported with letrozole, though not measured in the trial of adjuvant anastrozole. Further follow-up is awaited to determine the long-term cardiac effects of adjuvant letrozole in the BIG 1-98 trial.
Click to enlarge
Preferred Hormonal Agents for Older Women
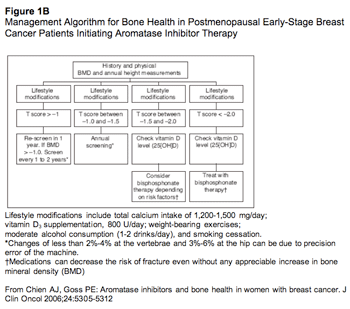
Thus, AIs are the preferred adjuvant hormonal agent for elderly women. Bone loss is a concern, and Figure 1 denotes an algorithm in managing bone health in postmenopausal women undertaking treatment with AIs, including a list of risk factors for osteoporosis.34 Recent reports of the Zometa-Femara Adjuvant Synergy Trial (Z-FAST) trial, which show up-front zoledronic acid (Zometa) therapy significantly slows bone loss in the lumbar spine, prompted use of preventive bisphosphonates. In this trial, women with early-stage breast cancer were randomized to receive up-front zoledronic acid therapy (4 mg intravenously every 6 months when letrozole therapy began) or delayed zoledronic acid (when bone mineral density of the lumbar spine or total hip T score decreased to less than −2.0 or when a nontraumatic fracture occurred).35 However, with only 1 year of follow-up, further trial maturity is warranted for efficacy and safety reports.
Click to enlarge
An upcoming trial, the Femara versus Anastrozole Clinical Evaluation (FACE) trial, will compare the two AIs as initial therapy for up to 5 years: letrozole (2.5 mg) and anastrozole (1 mg). This is a phase IIIb, open-label, randomized multicenter study with a target accrual of 4,000 postmenopausal women with hormone receptor-positive, node-positive breast cancer. The primary endpoint is disease-free survival, and secondary endpoints are overall survival, time to distant metastases, time to contralateral breast cancer, and breast cancer-specific survival. Evaluation of safety is also planned.36 Results of this trial will help us understand the differences in risk-benefit ratio of adjuvant treatment with letrozole and anastrozole.
Adherence to Adjuvant Hormonal Therapy
The benefit of adjuvant hormonal therapy can only be attained if the prescribed drug is taken. It has been reported that 17% of women over the age of 65 discontinued taking tamoxifen within the first 2 years,37 and 31% of women do not complete the 5-year course.38 Factors that predicted discontinuation were having neutral or negative beliefs about the value of tamoxifen, experiencing a side effect, and adding another prescription during the course of tamoxifen. Factors that indicated adherence were taking more prescription medications at baseline, viewing the use of tamoxifen in a positive light, and feeling more positive at follow-up about the use of tamoxifen.37,38
Thus, for older women, to improve adherence to prescription adjuvant hormonal therapy, we suggest that oncologists intervene by taking the following steps: educate women about the tremendous benefit of adjuvant hormonal therapy; continue to stress this benefit on follow-up (to offset the adverse symptom complaint rate of 60%-70%); review the medication list and usage at each visit; and elicit and address perceived side effects of treatment.
Conclusion
In women older than 75 years with hormone receptor-positive, T1N0 stage breast cancer and one or more comorbid illnesses, lumpectomy followed by adjuvant hormonal therapy, without radiation therapy, is an option. For the healthy 75 to 84-year-old woman without comorbid illness, we recommend that adjuvant radiotherapy be offered.
Adjuvant hormonal therapy should be discussed with all older women who have hormone receptor-positive breast cancer. Due to the lower toxicity and greater benefit of AIs compared with tamoxifen, AIs are the currently preferred adjuvant hormonal agents for postmenopausal women. However, increasing reports show a differing side-effect profile between anastrozole and letrozole; at this time, without results of a head-to-head comparison trial, anastrozole seems to have similar efficacy with less circulatory toxicity. Bone health with AIs is a concern and must be addressed at the outset, with screening for risk factors for osteoporosis, bone mineral density examinations, initiation of lifestyle modification interventions, and use of bisphosphonates when needed.
Finally, without adherence to adjuvant hormonal therapy, no benefit will be achieved. We should be aware of the possibility of nonadherence and initiate patient discussions to maximize adherence and treatment benefit.
CME Post-Test and Evaluation
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Smigal C, Jemal A, Ward E, et al: Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin 2006;56:168-183.
2. Edwards BK, Howe HL, Ries LA, et al: Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer 2002;94:2766-2792.
3. Crivellari D, Aapro M, Leonard R, et al: Breast cancer in the elderly. J Clin Oncol 2007;25:1882-1890.
4. Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G: Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol 2007;25:1858-1869.
5. Muss HB, Biganzoli L, Sargent DJ, Aapro M: Adjuvant therapy in the elderly: making the right decision. J Clin Oncol 2007;25:1870-1875.
6. Kimmick GG, Peterson BL, Kornblith AB, et al: Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol 2005;23:2201-2207.
7. Talarico L, Chen G, Pazdur R: Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004;22:4626-4631.
8. Satariano WA, Ragland DR: The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med 1994;120:104-110.
9. Swenson WT, Hoffman M, Hoffman A: Omission of radiotherapy after breast conserving surgery among elderly women with favorable prognosis tumors. J Clin Oncol 2007;25(18S):9034.
10. Truong PT, Bernstein V, Lesperance M, Speers CH, Olivotto IA: Radiotherapy omission after breast-conserving surgery is associated with reduced breast cancer-specific survival in elderly women with breast cancer. Am J Surg 2006;191:749-755.
11. Fisher B, Anderson S, Bryant J, et al: Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-1241.
12. Clark RM, McCulloch PB, Levine MN, et al: Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst 1992;84:683-689.
13. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2000;355:1757-1770.
14. Hughes KS, Schnaper LA, Berry D, et al: Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004;351:971-977.
15. Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG: Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst 2006;98:681-690.
16. Walter LC, Covinsky KE: Cancer screening in elderly patients: a framework for individualized decision making. JAMA 2001;285:2750-2756.
17. Wong ND, Thakral G, Franklin SS, et al: Preventing heart disease by controlling hypertension: impact of hypertensive subtype, stage, age, and sex. Am Heart J 2003;145:888-895.
18. Prescott RJ, Kunkler IH, Williams LJ, et al: A randomized controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess 2007;11:1-170.
19. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-1717.
20. Gail MH, Costantino JP, Bryant J, et al: Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst 1999;91:1829-1846.
21. Passage KJ, McCarthy NJ: Critical review of the management of early-stage breast cancer in elderly women. Intern Med J 2007;37:181-189.
22. Howell A, Cuzick J, Baum M, et al: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60-62.
23. Coates AS, Keshaviah A, Thurlimann B, et al: Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 2007;25:486-492.
24. Coombes RC, Kilburn LS, Snowdon CF, et al: Survival and safety of exemestane versus tamoxifen after 2-3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 2007;369:559-570.
25. Jakesz R, Jonat W, Gnant M, et al: Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005;366:455-462.
26. Goss PE, Ingle JN, Martino S, et al: A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793-1802.
27. Goss PE, Ingle JN, Martino S, et al: Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol 2007;25:2006-2011.
28. Crivellari D, Sun Z, Coates AS, et al: Aromatase inhibitors (AI) for elderly patients: efficacy, compliance and safety according to patient age in the BIG 1-98 trial. J Clin Oncol 2007;25(18S):9033.
29. Baum M, Budzar AU, Cuzick J, et al: Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002;359:2131-2139.
30. Buzdar AU, Guastalla JP, Nabholtz JM, Cuzick J, ATAC Trialists’ Group: Impact of chemotherapy regimens prior to endocrine therapy: results from the ATAC (Anastrozole and Tamoxifen, Alone or in Combination) trial. Cancer 2006;107:472-480.
31. The ATAC Trialists’ Group, Buzdar A, Howell A, et al: Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 2006;7:633-643.
32. Nevitt MC, Cummings SR, Stone KL, et al: Risk factors for a first-incident radiographic vertebral fracture in women > or = 65 years of age: the study of osteoporotic fractures. J Bone Miner Res 2005;20:131-140.
33. Silverman SL, Minshall ME, Shen W, et al: The relationship of health-related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum 2001;44:2611-2619.
34. Chien AJ, Goss PE: Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol 2006;24:5305-5312.
35. Brufsky A, Harker WG, Beck JT, et al: Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol 2007;25:829-836.
36. Jonat W, Mundhenke C: The FACE trial: letrozole or anastrozole as initial adjuvant therapy? Cancer Invest 2007;25:14-18.
37. Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA: Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol 2004;22:3309-3315.
38. Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA: Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 2006;99:215-220.