Recent Advances in the Management of Brain Metastases in Non-Small Cell Lung Cancer
Lung cancer is one of the most common and deadly malignancies in the United States, with an estimated 213,380 new cases in 2007 and an estimated 160,390 deaths in 2007. Approximately 85% of these patients will be diagnosed with non-small cell lung cancer (NSCLC), and only 10%-20% will have potentially curable disease.
Continuing Medical Education Information
Recent Advances in the Management of Brain Metastases in Non-Small Cell Lung Cancer
Activity Release Date: October 1, 2007
Activity Expiration Date: October 1, 2008
About the Activity
This activity is based on a brief article developed as part of the E-Update Series and posted on the Web. The series is geared to oncologists and addresses new treatments of cancer or modifications thereof.
This activity has been developed and approved under the direction of Beam Institute.
Activity Learning Objectives
After reading this article, participants should be able to:
Review the prominent role of whole-brain radiotherapy (WBRT) for patients with multiple brain metastases.Discuss the use of focal treatments, in combination with WBRT or alone, in terms of local control and survival.Identify several approaches to improving neurocognitive function in patients with brain metastases.Summarize the preliminary results of adding radiosensitizers such as motexafin gadolinium and efaproxiral as well as chemotherapy agents to radiotherapy for brain metastases.
Target Audience
This activity targets physicians in the fields of oncology and hematology.
Accreditation
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Beam Institute and The Oncology Group. Beam Institute is accredited by the ACCME to provide continuing medical education for physicians.
Continuing Education CreditAMA PRA Category 1 Credit™
The Beam Institute designates this educational activity for a maximum of 2 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Compliance Statement
This activity is an independent educational activity under the direction of Beam Institute. The activity was planned and implemented in accordance with the Essential Areas and policies of the ACCME, the Ethical Opinions/Guidelines of the AMA, the FDA, the OIG, and the PhRMA Code on Interactions with Healthcare Professionals, thus assuring the highest degree of independence, fair balance, scientific rigor, and objectivity.
However, Beam Institute, the Grantor, and CMPMedica shall in no way be liable for the currency of information or for any errors, omissions, or inaccuracies in the activity. Discussions concerning drugs, dosages, and procedures may reflect the clinical experience of the author(s) or may be derived from the professional literature or other sources and may suggest uses that are investigational in nature and not approved labeling or indications. Activity participants are encouraged to refer to primary references or full prescribing information resources. The opinions and recommendations presented herein are those of the author(s) and do not necessarily reflect the views of the provider or producer.
Financial Disclosures
Dr. Govindan receives research support from Bristol-Myers Squibb, Eli Lilly, Genentech, Pfizer, Sanofi-Aventis, and serves on the speakers' bureau for Eli Lilly and Genentech. Dr. Mehta is a consultant and speaker for Schering Plough. Dr. Siker has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Copyright
Copyrights owned by Beam Institute, a division of CME LLC. Copyright 2007, CME LLC. All rights reserved.
Contact Information
We would like to hear your comments regarding this or other activities provided by Beam Institute. In addition, suggestions for future activities are welcome. Contact us at:
Address:
Director of Continuing Education
Beam Institute
11 West 19th Street, 3rd Floor
New York, NY 10011-4280
Phone: 888-618-5781
Fax: 212-600-3050
e-mail:beaminstitute@cmp.com
Introduction
Lung cancer is one of the most common and deadly malignancies in the United States, with an estimated 213,380 new cases in 2007 and an estimated 160,390 deaths in 2007.1 Approximately 85% of these patients will be diagnosed with non-small cell lung cancer (NSCLC), and only 10%-20% will have potentially curable disease.
More than 25% of patients with lung cancer develop brain metastases, often the first site of recurrence, occurring more frequently in patients with adenocarcinoma and large cell carcinoma and in those with advanced disease. The incidence of brain metastases in general is believed to be rising due to changing patient demographics with a larger aging population at higher risk for developing malignancies, improved cancer treatments extending survival of patients and allowing the emergence of intracranial disease, new imaging modalities with better sensitivity for detecting occult disease, and the more frequent use of early magnetic resonance imaging (MRI) in staging asymptomatic patients.
Overall survival for patients with brain metastases remains poor. Historically, median survival with supportive care and symptomatic treatment has been only 1-2 months, whereas definitive treatment may extend survival to a median of 4 months. The Radiation Therapy Oncology Group (RTOG) has developed and validated a three-tiered prognostic classification system for patients with brain metastases using recursive partitioning analysis (RPA; Table 1).2,3 Favorable prognostic factors include a good performance status, a controlled primary tumor, age younger than 65 years, and metastases located in the brain only. Patients in RPA class 1 appear to have the longest median survival following whole-brain radiotherapy (WBRT), whereas patients in RPA classes 2 and 3 seem to survive for significantly shorter periods.
WBRT Alone
The appropriate use of WBRT can provide rapid attenuation of many neurologic symptoms, improve quality of life, and be especially beneficial in patients with large, multiple, or diffuse metastases, in patients whose lesions impinge on eloquent areas, and in patients with medical comorbidities that preclude them from surgery. Because multiple (more than one) metastases are found in approximately 80% of patients with brain metastases, WBRT is the treatment of choice for the vast majority of patients.
Since the use of WBRT was first described in 1954, many different dosing, timing, and fractionation schemes have been investigated; however, none of these trials has demonstrated superior survival with WBRT compared with conventional treatment.4-6, 10-14 Although the optimal schedule is still debated, typical WBRT regimens in the United States are 10 fractions of 3 Gy given over 2 weeks (30 Gy) or 15 fractions of 2.5 Gy given over 3 weeks (37.5 Gy). Because of the potential for increased risk of neurocognitive impairment in patients with prolonged survival, some clinicians prefer using schedules that employ prolonged administration times, such as 3 to 4 weeks, with a simultaneous reduction in dose per fraction, especially for patients with better prognoses. However, this approach has not yet been validated in prospective, randomized clinical studies.
A number of randomized trials have demonstrated improved local or locoregional control with conventional WBRT.2,5 At 1 year after WBRT alone, the actuarial local control rate has been found to be 0%-14% in randomized trials, suggesting that long-term control of brain metastases following conventional treatment is achieved in only a minority of patients.9,15 The majority of patients who are locally controlled die of extracranial disease, whereas patients with recurrent brain metastases typically succumb to central nervous system (CNS) disease.16
Complications of WBRT can be acute or delayed. Acute toxicities, appearing up to 90 days after treatment, associated with WBRT include alopecia, dermatitis, otitis externa, otitis media, hearing loss, nausea or vomiting, and somnolence. Most of these problems resolve relatively soon after the cessation of treatment. Late toxicities, occurring more than 90 days after treatment, may involve necrosis, personality changes, memory loss, cerebellar dysfunction, cataracts, and neurocognitive deterioration.
In addition to the toxicities of WBRT, the survival may be too short and meaningful palliation may not be achieved by enough patients to justify WBRT in all patients, although these data are relatively sparse. Nevertheless, when balancing the potential benefits of symptomatic relief, improvement in neurologic status, increased survival with limited toxicities (especially with proper dosage and modern fractionation schemes), and poor outcome without definitive treatment, WBRT remains the standard of care for patients with multiple brain metastases.
WBRT with Focal Treatment
Focal treatment combined with WBRT has been shown to improve overall survival in patients with a single metastasis based on prospective randomized trials.7-9 Improved surgical techniques and postoperative care have resulted in a decline in morbidity to less than 5%, making resection a safer option for patients with brain metastases. Surgery is indicated when tissue is needed to establish a diagnosis or to provide immediate palliation.
Level 1 evidence justifying the use of resection has been provided by two prospective randomized controlled studies; they have shown that resection followed by WBRT significantly improves survival in patients with a single brain metastasis (Table 2).8,9 Although a third phase III trial was unable to reproduce these results, this trial has been criticized due to the high proportion of patients with a poor performance status and extracranial disease compared with the former trials.17 These trials suggest that the benefit may be greatest in patients with stable disease, minimal systemic dissemination, younger age, and a good performance status.
Click to enlarge
The use of focal treatment in combination with WBRT in patients with more than one metastasis has not shown a survival benefit. Regarding the use of resection for patients with more than one brain metastasis, some preliminary data suggest that resection may increase survival in these patients; however, these data are retrospective, and this approach has not been validated in a prospective randomized trial.18
Stereotactic radiosurgery delivers precise, conformal radiation to a defined target in a single large dose through the use of multiple convergent beams. This technique allows for maximum sparing of surrounding normal tissue, with rapid dose falloff at the edge of the target volume. Primarily used to treat small lesions (less than 3 cm) in eloquent areas and residual disease after surgical resection, stereotactic radiosurgery is minimally invasive, well tolerated, and best suited for patients with a good performance status and limited extracranial disease. Potential adverse effects include brain swelling, nausea, dizziness, seizures, and headache, with radionecrosis appearing in approximately 5% of patients.19 In patients with brain metastases, local control rates range from 25%-100%, with a mean of 81%, and response rates vary from 30%-100%, with an average of 69%.20
The first two prospective randomized trials examining the use of radiosurgery with WBRT versus WBRT alone in patients with brain metastases demonstrated enhanced local control in the radiosurgery arm; however, these trials were criticized for poor design.15, 21 A larger, more recent multi-institutional RTOG trial confirmed these findings and showed superior survival in patients with a single metastasis treated with WBRT and radiosurgery compared with WBRT alone (6.5 vs 4.9 months; P = 0.04), providing level 1 evidence supporting this approach in these patients.7
Additionally, a multi-institutional retrospective review suggests that WBRT with radiosurgery may improve survival in patients with brain metastases in all three RTOG RPA classes.22 A higher performance status, a controlled primary tumor, absence of extracranial metastases, and a lower RPA predicted improved survival, suggesting that patients with these characteristics may benefit the most from radiosurgery.
Based on these trials, there is level 1 evidence to support the use of focal treatment with WBRT in patients with a single brain metastasis to maximize local control and prolong survival. The addition of these modalities in patients with more than one brain metastasis has yet to be defined. Only retrospective series examining the use of resection plus WBRT are available, whereas radiosurgery has only been shown to improve local control without a significant survival benefit in prospective trials. Retrospective trials suggest that certain patients with multiple metastases may benefit from the addition of radiosurgery; however, this approach cannot be recommended as standard of care at this time.23
A prospective study directly comparing resection and radiosurgery has not been completed, so a definitive conclusion regarding this issue is not currently possible. In the absence of level 1 evidence, the decision to use resection or radiosurgery in patients with a single brain metastasis should consider patient and tumor characteristics, such as medical comorbidities that may influence patients’ ability to tolerate resection, functional status, as well as the location and size of the intracranial lesion. Radiosurgery offers an alternative to resection and may be especially useful in patients who have a single lesion that impinges on an eloquent area or in those who are unable to tolerate surgery. Additionally, radiosurgery may be more cost-effective than resection.24 Resection may be preferred when the diagnosis is in doubt, when immediate relief of a major increase in intracranial pressure is necessary, and when the lesion is large (greater than 3-4 cm). For all patients, a team approach weighing the advantages and disadvantages of both options is recommended.
Focal Treatment Alone
With the increased efficacy of focal treatments, an approach using resection or stereotactic radiosurgery alone, with WBRT reserved for salvage, has been proposed, especially for younger patients with a good performance status and controlled extracranial disease. The rationale for this practice is to avoid neurocognitive impairment that may occur with WBRT. Opponents of this approach point out that even under ideal circumstances, microscopic disease may remain in the majority of patients receiving focal treatments and may rapidly emerge as symptomatic metastases. The aim of adjuvant WBRT is to eradicate any residual disease to enhance local control, both at the focal site and in the rest of the brain. Defining the best approach in regard to the timing and sequence of these modalities remains to be clarified and is the subject of the two studies summarized in Table 3.
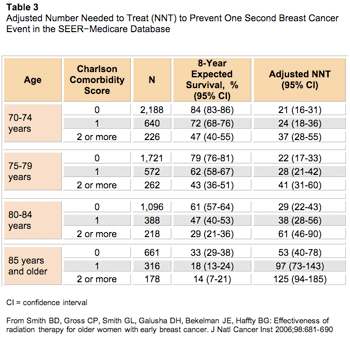
Click to enlarge
Patchell et al conducted the only randomized prospective trial examining the benefit of adjuvant WBRT following resection.25 In this study, 95 patients with a single brain metastasis were randomized to undergo resection with postoperative WBRT or resection with no postoperative WBRT. Patients who received postoperative WBRT had significantly decreased overall intracranial failure (18% vs 70%; P < 0.001), demonstrating a superior outcome with adjuvant WBRT. Advocates of focal treatment alone note that there was no difference between the two arms in regard to overall survival (48 vs 43 weeks;
P = 0.39). However, this trial was never powered to detect a change in the survival endpoint, and therefore using the survival endpoint from this trial to augment the argument to withhold WBRT is simply untenable.
Further confounding any potential conclusions, salvage WBRT was given to 61% of patients in the surgery-only arm. Additionally, the usage of salvage WBRT occurred very early, suggesting that no meaningful delay of WBRT usage was achieved. Although half of those patients who received surgery alone and WBRT at relapse died of CNS recurrence, only 14% of patients treated with surgery and immediate WBRT had CNS recurrence as a cause of death, implying that salvage WBRT is significantly inferior to up-front WBRT. It is important to note that patients who received both surgery and WBRT had a survival of 12 months, nearly two to three times longer than historic values.26
The Japanese Radiotherapy Oncology Group recently published the results of a multi-institutional, prospective, randomized trial investigating this question in patients with up to four brain metastases.27 In this study, 132 patients with lesions less than 3 cm were randomized to receive radiosurgery alone or radiosurgery followed by WBRT. Local control was significantly enhanced in patients treated with WBRT, with a 12-month intracranial recurrence rate of 46.8% in the WBRT plus radiosurgery arm versus 76.4% in the radiosurgery-alone arm (P < 0.001). The median survival was equivalent in both arms (7.5 vs 8.0 months; P = 0.42).
Opponents of the focal treatment-alone approach argue that most statisticians would consider the number of patients as inadequate to truly assess a survival difference. Additionally, salvage treatment was less frequently used in patients who received WBRT compared with radiosurgery alone. Death due to a neurologic cause occurred in 22.8% of patients in the WBRT plus radiosurgery arm, compared with 19.3% in the radiosurgery-alone arm (P = 0.64). Differences in the preservation of systemic and neurologic function and in radiation toxicities were not found, underscoring the fact that WBRT did not result in measurably greater neurologic deterioration. Another cooperative intergroup trial is also investigating this strategy in a prospective randomized trial that aims to accrue 480 patients over 5 years.
Those in favor of withholding WBRT contend that the comparable survival found in the trials previously described is enough to justify reserving WBRT for salvage despite the improved intracranial control with immediate adjuvant WBRT. The advocates of this practice propose that patients with limited disease (3-10 lesions, depending on the institution) be treated with focal treatment alone and be subsequently followed by serial imaging. If a new intracranial lesion appears, it should be treated with focal treatment again (usually radiosurgery). The purported high rate of neurotoxicity associated with WBRT provides the rationale for this approach.
DeAngelis et al suggested that the risk of developing dementia (based on a retrospective review of 47 patients treated with WBRT) was 11%.28 These patients were considered long-term survivors (more than 12 months). However, every patient who developed dementia received doses/fractionation higher than today’s standard. Of the 15 patients included in this report treated with regimens used today, none developed dementia.
Preventing recurrent disease has also been found to be important in preserving neurocognitive status and may outweigh the potential toxicities of WBRT. Patients with recurrent brain metastases have been found to have decreased mental performance and increased neurologic deficits.29,30 The trial by Aoyama et al previously reported that neurocognitive function in patients treated with radiosurgery alone was worse than that in those treated with radiosurgery followed by immediate adjuvant WBRT.31 Neurocognitive impairment represents a major issue in radiotherapy for patients with brain metastases and is discussed in the next section.
In summary, the importance of superior local control as demonstrated in patients receiving adjuvant WBRT cannot be understated, especially in patients with controlled systemic disease. Although survival has been found to be equivalent in prospective trials, the impact of salvage therapy with WBRT and the lack of appropriate power to detect a survival difference cannot be ignored. The risk of permanent adverse effects from WBRT must be weighed against the adverse effects that accompany tumor recurrence. Furthermore, serial imaging and retreatment with radiosurgery dramatically increase the overall cost of managing these patients. In the absence of evidence to substantiate claims that omission of WBRT results in decreased toxicity and improved quality of life, immediate adjuvant WBRT should remain the standard of care in the majority of patients receiving focal treatment.
Neurocognitive Function
With the advent of newer treatments prolonging survival and the relatively poor performance status and short overall survival found in the majority of patients with brain metastases, outcomes such as neurocognitive function and quality of life are important to consider. Neurocognitive dysfunction may be caused by radiotherapy, systemic treatment such as chemotherapy and hormonal agents, surgery, adjuvant medications, and the tumor itself.
Although the precise incidence of neurocognitive deficits in patients with brain metastases is unknown, one phase III trial of 401 patients reported baseline cognitive impairment in 91% of patients.32 In this trial, baseline neurocognitive function was demonstrated to be predictive of survival. Furthermore, recent trials have suggested that cognitive deterioration may be predictive of treatment failure, and those responding to WBRT (as measured on MRI) may have better neurocognitive function and longer survival than those not responding to WBRT.33,34
In the Japanese prospective randomized trial comparing WBRT and radiosurgery with radiosurgery alone for patients with one to four brain metastases, neurocognitive function was assessed using the Mini-Mental Status Examination (MMSE).31 Of the 82 patients with a baseline MMSE score of at least 27 or whose baseline MMSE score was no more than 26 but had improved to at least 27 after treatment, the 12- and 24-month actuarial rates of freedom from at least a 3-point drop in the MMSE were 76% and 69% in the WBRT plus radiosurgery group, compared with 59% and 52% in the radiosurgery-alone group, respectively. The average time to deterioration was 16.5 months in the WBRT plus radiosurgery group and 7.6 months in the radiosurgery-alone group (P = 0.05). This study suggests that, for most patients with brain metastases, control of the brain tumor is the most important factor for stabilizing neurocognitive function.
Approaches to improve neurocognitive function include pharmacologic agents and modifications to the delivery of WBRT. Agents such as methylphenidate and memantine (Namenda) have been used with modest results.35,36 Patients receiving doses of more then 3 Gy per fraction have been shown to be at higher risk for developing dementia in the long term, so hypofractionation regimens should be avoided.37 The hippocampus houses a neural stem cell population capable of generating migratory cells involved in repair and plasticity of brain function that has been demonstrated to be exquisitely radiosensitive in preclinical studies. A technique using conformal radiotherapy to avoid the hippocampus has been developed (Figure 1) and is under further investigation.38 This technology allows for full dose to the majority of the brain while limiting the dose to the hippocampus.
Click to enlarge
In summary, as some patients with brain metastases are surviving longer, understanding neurocognitive function has become increasingly important. Treatments with pharmacologic agents and modifications to radiotherapy are currently being examined in clinical trials. Further data are needed to validate these approaches.
Systemic Agents
Along with advances in the delivery of radiotherapy, the addition of systemic agents such as radiosensitizers and chemotherapy to radiotherapy is another strategy to improve treatment. Recently developed radiosensitizers such as motexafin gadolinium (MGd) and efaproxiral (RSR-13) have demonstrated initially promising results. These compounds are thought to have a synergistic effect when used in conjunction with radiotherapy; however, the optimal combination, including timing and sequence, is currently being investigated.
Click to enlarge
MGd is a redox modulator that enhances radiotherapy-induced apoptosis. This agent selectively concentrates in malignant tissue, as shown by MRI (Figure 2). Early studies showed response rates of 68%-72% in patients with brain metastases treated with MGd and WBRT. A phase III prospective randomized controlled trial compared WBRT alone and WBRT with MGd in 401 patients.39 There was no significant difference in the primary endpoints of median survival (5.2 vs 4.9 months; P = 0.48) and median time to neurologic progression (9.5 vs 8.3 months; P = 0.95). However, when tumor type was examined, an increased time to neurologic progression was found in the 63% of patients with NSCLC treated with MGd, compared with those who received WBRT alone (median not reached vs 7.4 months; P = 0.048). There was also a significant improvement in the secondary endpoints of death from CNS causes and memory and executive function. Adverse events were mild to moderate and easily manageable, and administration of MGd did not interfere with the delivery of radiotherapy.
A confirmatory phase III trial investigating the use of MGd in patients with brain metastases and NSCLC primary tumors has been completed, and results were presented at the 2006 American Society of Therapeutic Radiation Oncology annual meeting.40 In this international multicenter prospective trial, 554 patients were randomized to receive WBRT alone or WBRT with MGd. There was a trend toward an improvement in the primary endpoint, time to neurologic progression, in patients who received WBRT plus MGd that was not found to be significant (15.4 vs 10 months; P = 0.12).
On further analysis of patients by region, a significant prolongation of both time to neurologic and neurocognitive progression was observed in patients treated with WBRT and MGd in North America. Patients treated in North America received WBRT sooner after diagnosis of brain metastases than did patients treated in Europe and Australia (median, 1.6 vs 3.1 weeks), as patients outside North America were more often treated initially with chemotherapy. A significant improvement in time to neurologic progression was found in patients who received WBRT within 3 weeks of diagnosis of brain metastases regardless of the region, in patients whose lung cancer was newly diagnosed, in patients who never received chemotherapy prior to randomization, and in patients in RTOG RPA class II. Although MGd has shown promising results in a subset of patients, its precise role in the diagnosis and treatment of brain metastases remains undefined.
Efaproxiral interferes with the oxygen-binding affinity of hemoglobin, thereby increasing tissue pO2. Efaproxiral with WBRT was examined in 57 patients with brain metastases.41 These patients were compared retrospectively with a matched cohort from the RTOG RPA brain metastases database. The median survival was found to be significantly improved for the efaproxiral-treated patients (6.4 vs 4.1 months; P = 0.0174).
A prospective randomized trial did not confirm this survival benefit. In this phase III study, 515 patients with brain metastases in RTOG RPA class 1 or 2 were randomized to receive WBRT or WBRT with efaproxiral.42 No significant difference in the primary endpoint of survival was shown (5.4 vs 4.4 months; P = 0.16). Interestingly, on further analysis of the tumor type, patients with breast cancer (21%) demonstrated a significantly improved treatment effect with efaproxiral compared with WBRT alone, whereas patients with NSCLC did not show a treatment benefit. Further investigation of this observation is under way with the ENRICH trial, which recently completed enrolling women with breast cancer.
Combining chemotherapy with radiotherapy represents another multimodal approach to treating brain metastases. The timing of combination chemotherapy with WBRT varies with the region, with the majority of patients in North America receiving WBRT soon after diagnosis. Patients in Europe, especially France, often receive up-front chemotherapy.
This issue has been evaluated in a small phase III study by Robinet et al.43 A total of 176 patients with brain metastases from NSCLC were randomized to receive chemotherapy with cisplatin and vinorelbine followed by delayed or early WBRT. They found no significant difference in survival between the two arms (24 vs 21 weeks; P = 0.83), which is not surprising because of the high death rate due to extracranial progression in these patients.
Temozolomide (Temodar), an oral alkylating agent believed to have synergistic effects, has shown modest activity in patients with recurrent and newly diagnosed brain metastases. A large phase III trial by Antonadou et al randomized 134 patients to receive WBRT alone or WBRT plus concomitant and adjuvant temozolomide.44 The response rate was significantly improved in the temozolomide arm (53% vs 33%; P = 0.039). The benefit was significantly more pronounced in patients younger than age 60 and in those with a Karnofsky performance status of at least 90. There was no significant difference in survival (8.3 vs 6.3 months; P = 0.179) between the groups. These trials suggest a benefit with the addition of concomitant and adjuvant temozolomide to WBRT, but further data are needed to warrant treatment with temozolomide as standard practice.
In summary, the use of systemic agents and radiosensitizers has shown promise in early studies; however, further trials are needed to determine their use in patients with brain metastases. At this time, these agents should be used only in the setting of a clinical trial.
Prophylactic Cranial Irradiation
The role of prophylactic cranial irradiation (PCI), which has been beneficial in reducing the risk of brain metastases in patients with small cell lung cancer who achieved complete response to initial therapy, is still inadequately defined in patients with NSCLC. The rationale behind this approach is to eradicate occult microscopic disease before it manifests clinically, especially in high-risk patients or in those with better response to treatment, without causing severe adverse effects from treatment.
Four randomized trials have failed to show a survival benefit to PCI. A systematic review by the Cochrane collaboration analyzed the available data and concluded that there was insufficient evidence to support the use of PCI as standard practice.45 However, the available trials showed a reduction in the incidence of brain metastases in patients, justifying further investigation of PCI. PCI has been proposed as an approach in those at high risk for developing brain metastases, such as those with complete or significant response to initial therapy (approximately 40%) and those with larger tumors, advanced tumor stage, as well as adenocarcinoma and tumors of undifferentiated histologies.46,47 An intergroup prospective controlled trial, aiming to recruit more than 1,000 patients, is ongoing. These patients will be randomized to receive observation or PCI following planned treatment, and data on quality of life and toxicity in addition to the primary endpoint of survival will be collected.
Conclusion and Future Directions
Despite advances in treatment delivery, a dramatic improvement in outcome has not been shown in patients with brain metastases from NSCLC. Definitive WBRT remains the standard of care for the majority of patients, especially those with multiple lesions. For patients with a single metastasis, large phase III trials have shown a survival benefit in those treated with resection or radiosurgery. In the absence of a prospective controlled trial comparing radiosurgery and resection, definitive recommendations cannot be made on the indications for either modality; however, radiosurgery is preferred in patients with tumors impinging on eloquent areas or in those with medical comorbidities precluding them from surgery. Resection may be preferred when tissue is needed for histologic confirmation of disease, immediate relief of increased intracranial pressure is necessary, or patients have large (greater than 3-4 cm) lesions. These recommendations are supported by the results of two recent meta-analyses.48,49
Although focal treatments such as resection and radiosurgery have shown numerically similar survival compared with focal treatment plus WBRT, these trials were not adequately powered for the survival endpoint, local control improved substantially with the addition of WBRT, and neurocognitive function was worse in the radiosurgery-alone arm. Until there is evidence that the omission of WBRT results in an improved clinical outcome, immediate adjuvant WBRT should remain the standard of care in patients receiving local treatment.
Future directions in the management of brain metastases in patients with NSCLC include trials investigating quality-of-life measures such as neurocognitive function. Strategies to improve neurocognitive function, such as pharmacologic agents and modifications in the delivery of radiotherapy, are under investigation. Furthermore, the application of multimodal approaches involving systemic agents such as radiosensitizers and chemotherapy has demonstrated promising results and is the subject of ongoing research. Further data are awaited before routine administration of these approaches is recommended.
CME Post-Test and Evaluation