Chemotherapy-induced nausea and vomiting (CINV) remains an important adverse effect of cancer therapy. The goal of CINV prophylaxis is to reduce the morbidity associated with nausea and vomiting, as well as to preserve quality of life, while maintaining the desired chemotherapy regimen. The US Food and Drug Administration has recently approved new therapies for prevention of CINV, including the neurokinin-1 (NK1) receptor antagonist rolapitant and the fixed-dose combination of the second-generation 5-hydroxytryptamine type 3 receptor antagonist palonosetron with the novel NK1 receptor antagonist netupitant. Alternative agents, like the atypical antipsychotic olanzapine, have also expanded the options available for preventing delayed and refractory CINV. Consensus guidelines for prevention of CINV from several organizations are generally consistent with one another and are updated based on expert review of available clinical trial data. This article will address changes in CINV guidelines over the past 5 years and provide updates on recently approved agents and agents that are expected to be approved, based on published phase III trials. It will also explore other factors affecting optimal CINV control, including the role of patient-related risk factors and the role of physician adherence to antiemetic guidelines in reducing the residual risk of CINV.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) remains an important adverse effect of cancer therapy. Prevention of CINV is foundational in providing optimal patient-centered cancer care. Recent advances in understanding the pathophysiology of CINV, which involves multiple neurotransmitter and receptor systems, have led to better understanding of the role of older available targeted agents and facilitated the development of new agents. Consensus guidelines for prophylaxis of CINV are available from the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO). Their recommendations for effective prophylaxis for CINV are primarily based on the emetogenic potential of specific chemotherapeutic agents, which is broadly divided into four categories: highly emetogenic chemotherapy (HEC; > 90% risk of emesis), moderately emetogenic chemotherapy (MEC; > 30%–90% risk), low emetogenic chemotherapy (LEC; 10%–30% risk), and minimal emetogenic chemotherapy (< 10% risk).[1-3]
Certain chemotherapeutic agents-for example, cisplatin-have a biphasic pattern of associated CINV symptom development, with an initial peak within 1 to 2 hours after chemotherapy administration and a second peak at 48 to 72 hours that generally resolves within 5 days. These two phases of CINV are defined as acute (≤ 24 hours after chemotherapy) and delayed (> 24–120 hours).[4] Signaling by serotonin (5-hydroxytryptamine) or 5-hydroxytryptamine type 3 (5-HT3) primarily mediates the acute phase, whereas the neurokinin-1 (NK1) signaling pathway mediates the delayed phase[5]; however, there is evidence for interaction between these pathways as well.[6,7]
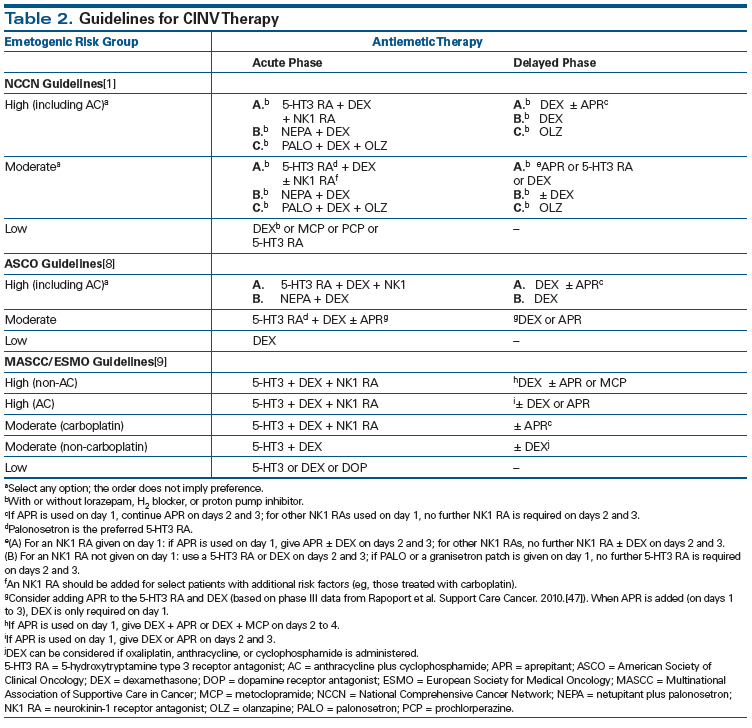
ASCO published antiemetic guidelines for CINV management initially in 1999, with updates in 2006, 2011, and 2015.[8] NCCN guidelines are updated most frequently, with the most recent version on CINV released in March 2016 (Version 2.2016).[1] MASCC/ESMO guidelines were also updated in March 2016 after the Copenhagen Consensus Conference on Antiemetic Therapy held in June 2015[9]; publication is forthcoming. These guidelines are generally consistent with one another. They are updated based on expert review of available clinical trial data comparing different regimens for CINV prophylaxis. The primary endpoint in these clinical trials is typically complete response (CR), defined as no emesis and no use of rescue medication during the acute, delayed, and overall phases (0–120 hours). Current CINV guidelines also address the emetogenic potential of oral chemotherapeutic agents and biologics, as well as the newer categories of cancer drugs.
Updates on NK1 Receptor Antagonists
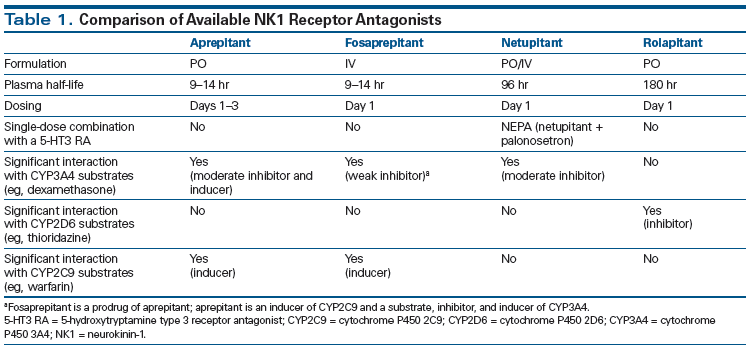
A role for NK1 receptors in suppression of the emetic reflex was first identified in the 1990s.[10] Subsequent studies showed a potent antiemetic effect with selective antagonists of the NK1 receptors. Since the US Food and Drug Administration (FDA) approval of the first NK1 receptor antagonist (NK1 RA), aprepitant, in 2003, new agents in this class with distinct properties have been approved for CINV prophylaxis.
Netupitant administered in combination with palonosetron as NEPA
Netupitant is a highly selective NK1 RA that exhibits a high degree of receptor occupancy in vitro and in vivo.[11] The plasma half-life of netupitant is approximately 96 hours, making single-prophylaxis dosing appropriate.[8] It is a substrate and moderate inhibitor of cytochrome P450 3A4 (CYP3A4).[12] Unlike with aprepitant, no clinically relevant interactions with oral contraceptives or warfarin have been noted with netupitant (Table 1).[13] Synergy of netupitant with palonosetron has been documented in vitro.[7,14] NEPA is an oral, single-dose, single-capsule, fixed-combination antiemetic agent containing netupitant (the NK1 RA) and palonosetron (the 5-HT3 RA). The safety and efficacy of NEPA have been demonstrated in three randomized trials involving chemotherapy-naive patients predominantly affected by solid tumors.
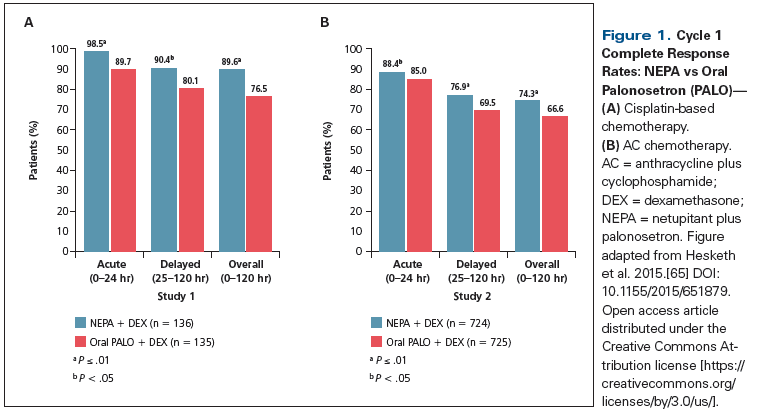
In a phase II dose-ranging study, 694 patients receiving HEC (cisplatin-containing chemotherapy) were randomized to five different therapeutic arms; these included netupitant administered at three different doses (100, 200, or 300 mg) plus palonosetron at 0.5 mg PO (NEPA100, NEPA200, NEPA300), palonosetron only (0.5 mg PO), or an exploratory treatment arm comprising aprepitant (the standard 3-day regimen) plus ondansetron (32 mg IV).[15] All patients received dexamethasone on days 1 to 4. The primary study endpoint was CR during the overall phase. NEPA was superior to palonosetron alone at all doses, during the acute, delayed, and overall phases; the highest NEPA dose yielded the greatest benefit (CR rates: 87.4%, 87.6%, and 89.6% for NEPA100, NEPA200, and NEPA300, respectively, vs a CR rate of 76.5% for patients randomized to palonosetron monotherapy; P < .05). Although no formal comparisons were intended, the results with NEPA300 were numerically better than those achieved with the aprepitant-ondansetron regimen for all efficacy endpoints and time intervals. Adverse events were comparable across groups. Based on these findings, NEPA300 was selected for phase III trials.
The efficacy of NEPA300 vs palonosetron (0.5 mg) was evaluated in a phase III study involving 1,455 patients treated with cyclophosphamide plus an anthracycline chemotherapy (doxorubicin or epirubicin); all patients received dexamethasone on day 1.[16] NEPA300 produced a significant improvement in CR rates compared with palonosetron (Figure 1) during the acute phase (88.4% vs 85.0%; P = .047), delayed phase (76.9% vs 69.5%; P = .001), and overall phase (74.3% vs 66.6%; P = .001). NEPA300 was well tolerated and the safety profile was comparable between the two treatment groups; headache (3.3% with NEPA300 and 3% with palonosetron) and constipation (2.1% in both study arms) were the most common treatment-related adverse events.
Another phase III trial, involving 413 patients, evaluated the safety and efficacy of NEPA300 administered over multiple cycles of therapy with a variety of HEC or MEC regimens; all patients received dexamethasone on days 1 to 4 for HEC and on day 1 for MEC.[17] A reference arm utilizing palonosetron and aprepitant was included. NEPA300 showed superior and persistent CR rates over repeated cycles of chemotherapy (CR rate for cycle 1: NEPA300, 81%; aprepitant, 76%). NEPA300 retained efficacy and safety over multiple cycles of HEC and MEC. No new safety signals were identified after repetitive use. Based on these data, in October 2014 the US Food and Drug Administration (FDA) approved NEPA (at the 300-mg dose of netupitant) for prevention of CINV.
Special considerations and key points regarding the use of NEPA are as follows:
• NEPA is a single-dose, fixed-combination, oral regimen with dual 5-HT3/NK1 receptor inhibition that offers increased convenience for patients and the potential to improve adherence to CINV guidelines.
• NEPA300 (300-mg netupitant/0.5-mg palonosetron) is superior to palonosetron alone (0.5 mg PO) in prevention of CINV during the acute, delayed, and overall phases in patients treated with HEC.
• NEPA is safe and effective over repeated cycles of HEC or MEC.
• Like aprepitant, netupitant is a substrate and moderate inhibitor of CYP3A4; therefore, it can potentially interact with drugs like dexamethasone that are substrates of CYP3A4. The dose of dexamethasone should be reduced (administered at 12 mg) when dexamethasone is used in combination with NEPA.
• NEPA does not have clinically relevant interactions with oral contraceptives or cytochrome P450 2C9 substrates (eg, warfarin).
In a phase II dose-ranging study, 694 patients receiving HEC (cisplatin-containing chemotherapy) were randomized to five different therapeutic arms; these included netupitant administered at three different doses (100, 200, or 300 mg [NEPA100, NEPA200, NEPA300]) plus palonosetron (0.5 mg PO), palonosetron only (0.5 mg PO), or an exploratory treatment arm comprising aprepitant (the standard 3-day regimen) plus ondansetron (32 mg IV).[15] All patients received dexamethasone on days 1 to 4. The primary study endpoint was CR during the overall phase. NEPA was superior to palonosetron alone at all doses, during the acute, delayed, and overall phases; the highest NEPA dose yielded the greatest benefit (CR rates: 87.4%, 87.6%, and 89.6% for NEPA100, NEPA200, and NEPA300, respectively, vs a CR rate of 76.5% for patients randomized to palonosetron monotherapy; P < .05). Although no formal comparisons were intended, the results with NEPA300 were numerically better than those achieved with the aprepitant-ondansetron regimen for all efficacy endpoints and time intervals. Adverse events were comparable across groups. Based on these findings, NEPA300 was selected for phase III trials.
Rolapitant
Rolapitant is a highly selective, long-acting, oral NK1 RA. It has high binding affinity towards the NK1 receptor, with > 90% receptor binding for up to 5 days.[18] It also has a long plasma elimination half-life of about 180 hours, suggesting that this drug can provide CINV prophylaxis during both the acute and delayed phases. Rolapitant is distinct from netupitant and other NK1 RAs in that it does not induce or inhibit CYP3A4.[19] Three randomized controlled trials conducted in chemotherapy-naive patients have demonstrated the safety and efficacy of rolapitant in CINV prophylaxis.
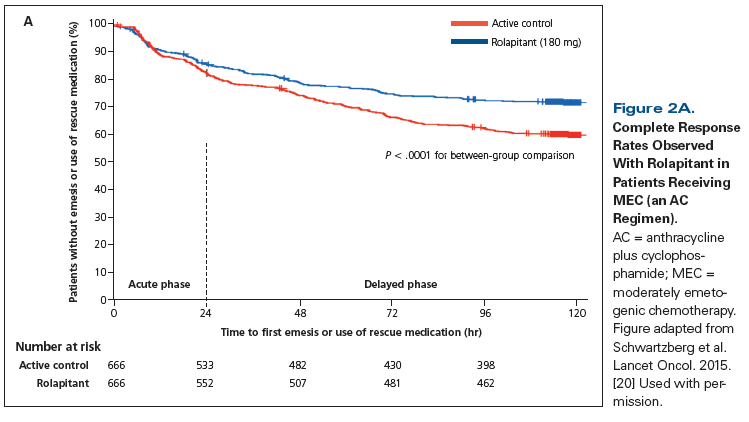
In a phase III trial, 1,369 patients receiving MEC or anthracycline plus cyclophosphamide (AC) chemotherapy were randomized to a study group receiving rolapitant (180 mg PO) or an active control group receiving placebo; all patients received granisetron (10 µg/kg IV) on day 1 and dexamethasone (20 mg on day 1 followed by 8 mg twice daily on days 2–4).[20] The primary endpoint was CR during the delayed phase in cycle 1. The rolapitant group showed significant improvement in CR rates compared with the active control group (Figure 2A) during the delayed phase (71% vs 62%; P < .0002) and overall phase (69% vs 58%; P < .0001). However, no significant difference was seen during the acute phase (83% vs 80%; P = .14). The quality-of-life score was significantly better for patients treated with rolapitant vs the active control (73% vs 67%; P = .02). Of note, at the time of study design, AC chemotherapy was considered MEC, with standard therapy at that time being a 5-HT3 RA plus dexamethasone (comprising the active control group).
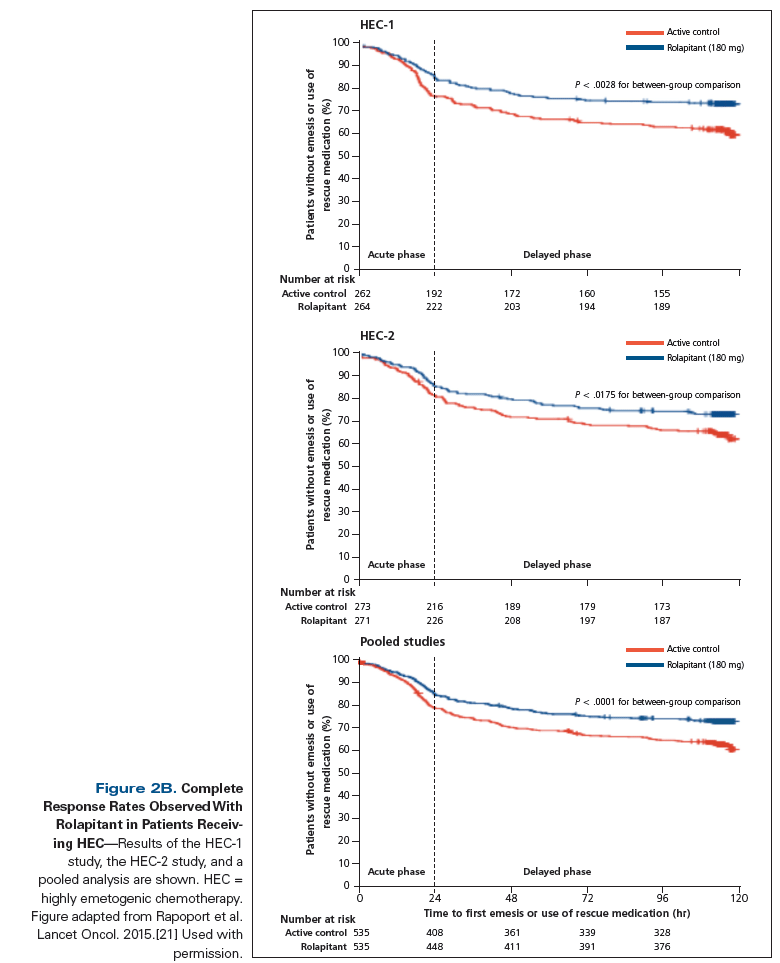
Rapoport et al conducted two phase III trials in patients receiving HEC (HEC-1 and HEC-2).[21] Patients were randomized to a rolapitant group (with the drug administered at 180 mg PO) and an active control group (placebo); all patients received granisetron (10 μg/kg IV) and dexamethasone (20 mg orally on day 1 and 8 mg twice daily on days 2–4). Pooled analyses of both studies, along with individual study results, were reported. The rolapitant group showed significant improvement in the CR rate during the delayed phase, compared with the active control group (HEC-1: 73% vs 58%, respectively, P = .0006; HEC-2: 70% vs 62%, P = .04; pooled studies: 71% vs 60%, P = .0001). In HEC-1 and pooled studies, a significantly greater proportion of patients in the rolapitant group achieved CR in the acute phase; however, in HEC-2, there were no significant differences between the two groups in the acute phase (Figure 2B). The incidence of adverse events was similar across treatment groups. The most common treatment-related adverse events were headache and constipation (< 1%). In September 2015, the FDA approved rolapitant for the prevention of delayed-phase CINV in patients being treated with MEC or HEC.
Special considerations and key points regarding the use of rolapitant are as follows:
• Rolapitant is a highly selective, long-acting, oral NK1 RA for prevention of CINV in patients being treated with MEC or HEC.
• Rolapitant is effective over multiple cycles of therapy.
• Rolapitant does not induce or inhibit CYP3A4 like other NK1 RAs, and it does not require dose adjustments of concomitantly administered drugs metabolized by CYP3A4, particularly dexamethasone.
• Rolapitant does inhibit cytochrome P450 2D6, which is involved in the metabolism of certain drugs, such as thioridazine.
Updates on 5-HT3 RAs
The first-generation 5-HT3 RAs (ondansetron, granisetron, and dolasetron) have revolutionized CINV prevention since their initial FDA approval in 1991. Several comparative trials have shown similar efficacy among first-generation 5-HT3 RAs, with CR rates for HEC reaching 70% during the acute phase.[22] They are substantially less effective at preventing delayed-phase CINV.[23] However, newer 5-HT3 antiemetics have shown an advantage over the older agents in preventing CINV.
TO PUT THAT INTO CONTEXT
[[{"type":"media","view_mode":"media_crop","fid":"51123","attributes":{"alt":"","class":"media-image","id":"media_crop_3157207535551","media_crop_h":"0","media_crop_image_style":"-1","media_crop_instance":"6291","media_crop_rotate":"0","media_crop_scale_h":"0","media_crop_scale_w":"0","media_crop_w":"0","media_crop_x":"0","media_crop_y":"0","style":"height: 145px; width: 144px;","title":" ","typeof":"foaf:Image"}}]]
David Warr, MD
Princess Margaret Cancer Centre at University Health Network
Toronto, Ontario, CanadaWhat Factors Should Be Considered Regarding Antiemetic Therapy for Patients at Risk for CINV?Joint guidelines from the Multinational Association of Supportive Care in Cancer and the European Society for Medical Oncology on management of chemotherapy-induced nausea and vomiting (CINV) no longer identify palonosetron as a preferred 5-hydroxytryptamine type 3 receptor antagonist (5-HT3 RA) in this setting. Although single-agent palonosetron is superior to single-day ondansetron or granisetron, it has not shown statistical superiority in studies with control arms using guideline-adherent antiemetics. Economic incentives that encouraged the use of IV palonosetron in the United States discourage its use elsewhere. Will generic palonosetron change this?Rolapitant, the newest-generation neurokinin-1 receptor antagonist (NK1 RA), has a 10-fold longer half-life than aprepitant and, unlike aprepitant, does not inhibit cytochrome P450 3A4 (CYP3A4). However, since even a single dose of fosaprepitant is effective, and no interaction with intravenous CYP3A4-metabolized chemotherapy drugs has been observed, it is unclear how rolapitant would prove advantageous. Administration of a single dose of rolapitant for patients receiving 3 to 5 days of treatment with cisplatin should be tested.Olanzapine, an atypical antipsychotic, has demonstrated impressive activity against nausea and vomiting. Will sedation (reported in 70% of patients)[1,2] relegate it to second-line use in countries that can afford an NK1 RA? Would doses lower than 10 mg be effective, with the advantage of less sedation?What Challenges Remain?Patient risk factors for CINV have not concretely influenced guidelines. Three attempts to identify a truly low-risk group among those receiving highly emetogenic chemotherapy have failed.[3-5] If there is a place for consideration of risk factors, they will be used best to identify patients about to receive moderately emetogenic chemotherapy who should be treated with either an NK1 receptor antagonist or olanzapine in addition to a 5-HT3 RA and a corticosteroid.Remarkable progress has been made in our quest to prevent CINV. The biggest remaining challenge is to ensure more widespread use of evidence-based guidelines in clinical practice.Financial Disclosure:Dr. Warr is a consultant to Helsinn and Tesaro, and he serves on the speakers bureau of Merck.REFERENCES1. Tan L, Liu J, Chen J, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res. 2009;28:131.2. Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer. 2016;24:675-82.3. Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer. 2010;18:1171-7.4. Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer. 2011;19:807-13.5. Warr DG. Can patient risk factors outperform antiemetic guidelines? Choosing wisely. JAMA Oncol. 2016;2:232-3.
Palonosetron
Palonosetron is a second-generation 5-HT3 RA that differs from other 5-HT3 RAs in several pharmacologic characteristics, including high affinity for the 5-HT3 receptor (approximately 10-fold higher than first-generation 5-HT3 RAs). The plasma half-life of palonosetron is approximately 40 hours, as compared with 5 to 12 hours for first-generation 5-HT3 RAs.[24] Palonosetron acts as an allosteric antagonist, inducing a conformational change in 5-HT3 receptors, and has been associated with receptor internalization, reducing receptor density at the cell surface.[7,25] The safety and efficacy of palonosetron have been demonstrated in multiple randomized trials. Two randomized phase III studies demonstrated superior efficacy of palonosetron vs first-generation 5-HT3 RAs in patients receiving MEC and AC chemotherapy.[26,27] Chemotherapy-naive as well as non-naive patients were included in these studies.
Another phase III trial compared palonosetron (at 0.25 mg and 0.75 mg IV) vs ondansetron (at 32 mg IV) predominantly in patients with lung and ovarian cancer treated with HEC (cisplatin, 83%, and cyclophosphamide, 25%); two-thirds of patients received corticosteroids in addition to the 5-HT3 RA.[28] CR rates were numerically better for both doses of palonosetron but were not statistically significant during all CINV phases. However, patients receiving concomitant dexamethasone and 5-HT3 RA showed significantly better CR rates during the acute and delayed phases if they received palonosetron at 0.25 mg. Suppression of emesis during the delayed and overall phases was also significantly better for patients treated with palonosetron.
The efficacy of palonosetron in combination with dexamethasone was evaluated in a phase III trial (n = 1,114) comparing palonosetron (0.75 mg IV) vs granisetron (40 μg/kg IV) in predominantly chemotherapy-naive patients receiving HEC (cisplatin, 57%; AC, 43%).[29] The coprimary endpoint was noninferiority of CR rates during the acute phase and superiority during the delayed phase. Palonosetron was noninferior to granisetron during the acute phase (75.3% vs 73.3%) and was statistically superior in the delayed phase (56.8% vs 44.5%; P < .0001) and overall phase (51.5% vs 40.4%; P = .0001). Similar results were reported in follow-up trials of HEC[30] and MEC.[31]
A pooled analysis of outcomes from these four randomized trials (N = 2,962) with MEC and HEC regimens demonstrated a statistically significant improvement in CR rates for palonosetron vs a first-generation 5-HT3 RA during both the delayed phase (57% vs 45%, respectively; P < .0001) and the overall phase (51% vs 40%; P < .0001), but not in the acute phase (69% vs 66%; P = .091).[32] Based on these trials, the FDA approved palonosetron for prevention of acute and delayed CINV associated with initial and repeat courses of MEC and HEC.
Special considerations and key points regarding the use of palonosetron are as follows:
• Palonosetron differs pharmacologically and clinically from first-generation 5-HT3 RAs.
• Palonosetron is superior to first-generation 5-HT3 RAs in the prevention of CINV in patients receiving MEC or HEC during the delayed and overall phases.
• A 0.5-mg PO dose of palonosetron has been established as therapeutically equivalent to a 0.25-mg IV dose.[33,34] The oral formulation of palonosetron is available as a single agent outside the United States. Oral palonosetron is also available as part of NEPA, the fixed-dose combination of palonosetron with netupitant.
• Although the cost of treatment with palonosetron is higher than that of first-generation 5-HT3 RAs, it is associated with less healthcare service utilization in management of CINV, leading to a lower overall cost of utilization.[35]
• Combination of an NK1 RA with palonosetron and dexamethasone increases CR rates.
APF530
APF530 is a novel, extended-release, subcutaneous granisetron formulation. This drug utilizes a viscous bioerodible Biochronomer delivery system to provide sustained release of therapeutic concentrations of granisetron for ≥ 5 days.[36] APF530 comprises 2% granisetron and a polymer vehicle of tri(ethylene glycol) poly(ortho ester) (TEG-POE) that undergoes controlled hydrolysis, resulting in slow, controlled, and sustained drug release.
In phase I/II pharmacokinetic studies, after repeated IV doses of granisetron (50 μg/kg) at 24 and 48 hours, plasma concentrations of granisetron were noted to be 3.67 ng/mL and 0.89 ng/mL, respectively. In contrast, the concentration of granisetron at 168 hours (7 days) after a single dose of APF530 (500 mg) was 1.96 ng/mL, showing sustained therapeutic concentration of granisetron for prolonged duration after APF530 administration.[37]
In a phase III trial, 1,404 patients receiving MEC or HEC (MEC group, n = 669; HEC group, n = 735) were randomized 1:1:1 to receive single-dose APF530 at 250 mg or 500 mg, or palonosetron at 0.25 mg IV; dexamethasone was administered per protocol.[38] The primary objective was to establish APF530 noninferiority to palonosetron during the acute phase for patients treated with MEC or HEC and during the delayed phase for those receiving MEC, and to determine whether APF530 is superior to palonosetron during the delayed phase for patients receiving HEC. Both doses of APF530 (250 mg and 500 mg) were noninferior to palonosetron during the acute phase after MEC (CR rates, 74.8% and 76.9%, respectively, vs 75.0% for palonosetron) and HEC (CR rates, 77.7% and 81.3%, respectively, vs 80.7% for palonosetron). APF530 at 500 mg was also noninferior to palonosetron during the delayed phase after MEC (CR rate, 58.5% vs 57.2%). However, APF530 was not superior in preventing delayed CINV after HEC.
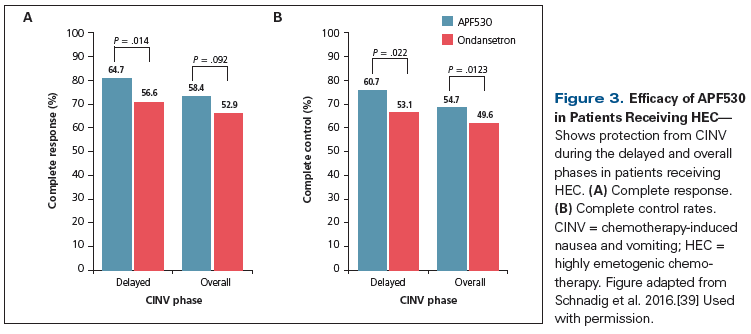
Another phase III study (n = 942) of patients receiving HEC compared APF530 (500 mg) vs ondansetron (0.15 mg/kg IV; maximum dose, 16 mg); all patients received fosaprepitant (150 mg IV) and dexamethasone (12 mg IV, days 1–4).[39] Most patients received an AC-based regimen or cisplatin. The CR rate was significantly greater in the APF530 group compared with the ondansetron group (Figure 3) during the delayed phase (64.7% vs 56.6%, respectively; P = .014) and numerically higher in the overall phase (58.4% vs 52.9%, respectively; P = .092) and the acute phase (75% vs 73%; P = .502). The rate of complete control (no emesis, no worse than mild nausea, and no use of rescue medications) during the delayed phase was also significantly higher in the APF530 group. Patient-reported satisfaction with control of CINV was significantly better for those treated with APF530 in the delayed phase (P = .04). The most common treatment-related adverse events were constipation, fatigue, headache, and injection site reactions. These studies suggest that APF530 is a potential alternative agent for CINV prophylaxis in patients treated with MEC and HEC.
Special considerations and key points regarding the use of APF530 are as follows:
• APF530 is a novel, extended-release, subcutaneous formulation of granisetron that utilizes the bioerodible Biochronomer delivery system to provide sustained release of therapeutic concentrations of granisetron for ≥ 5 days.
• The APF530 250-mg subcutaneous dose is equivalent to granisetron at 5 mg IV, and the 500-mg dose is equivalent to granisetron at 10 mg IV.
• APF530 is noninferior to IV palonosetron in preventing acute CINV after MEC or HEC.
• APF530 is superior to IV ondansetron in combination with fosaprepitant and dexamethasone for prevention of CINV after HEC in the delayed phase.
• APF530 is generally well tolerated, with no new or unexpected safety findings.
Development of Olanzapine
Olanzapine (a thienobenzodiazepine) is an atypical antipsychotic that blocks multiple neurotransmitters[40] and interacts with D2, 5-HT2C, and 5-HT3 receptors, which are involved in nausea and emesis.[41]
Olanzapine for management of breakthrough CINV
Breakthrough CINV is defined as vomiting and/or nausea within 5 days of chemotherapy administration despite appropriate antiemetic treatment based on CINV guidelines. The incidence of breakthrough CINV is in the range of 30% to 50% in patients receiving MEC or HEC. Management of breakthrough CINV is often challenging and requires additional rescue medication, preferably from a different drug class. However, no single treatment is better than the others for managing breakthrough emesis.[1]
The efficacy of olanzapine for breakthrough CINV was evaluated in a phase III trial that included 276 chemotherapy-naive patients who received HEC with appropriate CINV prophylaxis (fosaprepitant, palonosetron, and dexamethasone in combination). These patients were randomized to group 1 (olanzapine at 10 mg PO daily for 3 days if the patient developed breakthrough CINV) or group 2 (metoclopramide at 10 mg orally 3 times daily for 3 days if the patient developed breakthrough CINV).[40] The authors reported that 42% of patients in group 1 and 39% of patients in group 2 developed breakthrough CINV and were treated with olanzapine or metoclopramide, respectively. During the 72-hour observation period, the rate of no emesis was significantly better for patients treated with olanzapine, compared with those who received metoclopramide (70% vs 31%, respectively; P < .01). Olanzapine was also significantly better in controlling nausea (68% vs 23%, respectively; P < .01). There were no grade 3 or 4 toxicities. Based on this study, the NCCN guidelines were updated to include olanzapine as a treatment option for breakthrough CINV.
Olanzapine as an alternative to NK1 RAs for CINV prophylaxis
A phase III study by Navari et al evaluated the efficacy of olanzapine (10 mg PO on days 1–4) vs a standard 3-day aprepitant regimen for prevention of CINV; the study included chemotherapy-naive patients receiving HEC (cisplatin, or cyclophosphamide plus anthracycline), and all patients received palonosetron (0.25 mg IV on day 1) and dexamethasone.[42] The CR rates were not statistically different between olanzapine and the aprepitant group during the acute phase (97% vs 87%, respectively), delayed phase (77% vs 73%), and overall phase (77% vs 73%); nausea was better controlled in the olanzapine group. There were no grade 3 or 4 toxicities. NCCN guidelines have included olanzapine as an alternative to NK1 RAs in combination with the 5-HT3 RA palonosetron and dexamethasone in controlling CINV in patients receiving HEC or MEC.
Special considerations and key points regarding the use of olanzapine are as follows:
• Olanzapine is superior to metoclopramide for treatment of breakthrough CINV in patients receiving HEC.
• Olanzapine can be selected as an alternative to treatment with an NK1 RA in combination with a 5-HT3 RA and dexamethasone in patients receiving HEC or MEC.
• Olanzapine should be used with caution in elderly patients and patients with type 2 diabetes and hyperglycemia.[43]
• Common side effects of olanzapine include sedation and weight gain, and use of this agent is associated with the onset of diabetes mellitus.
Significant Updates in Guidelines for Antiemetic Prophylaxis Against CINV
There have been several important updates in the last 5 years to guidelines for antiemetic therapy as prophylaxis against CINV (Table 2).[1,8,9] Key changes include the reclassification of the AC regimen as HEC (previously classified as MEC); the addition of olanzapine as a treatment option for CINV; expanded use of antiemesis treatment with NK1 RAs and 5-HT3 RAs in patients receiving carboplatin-based chemotherapy; and the addition of newer biologics and oral chemotherapeutics.
Reclassification of AC as HEC
In a November 2011 update to the ASCO guidelines, AC chemotherapy was reclassified as HEC.[3] Currently, AC regimens are also recognized as HEC in the NCCN and MASCC/ESMO guidelines for the management of CINV.[1,9] The guidelines recommend that patients treated with AC receive a three-drug regimen (NK1 RA, 5-HT3 RA, and dexamethasone) for CINV prophylaxis.
Addition of olanzapine as an antiemetic option for prevention of CINV
NCCN guidelines now include olanzapine for treatment of breakthrough CINV and as an alternative to NK1 RA in combination with a 5-HT3 RA and dexamethasone for prevention of CINV in patients receiving HEC or MEC.[1]
CINV prophylaxis for carboplatin
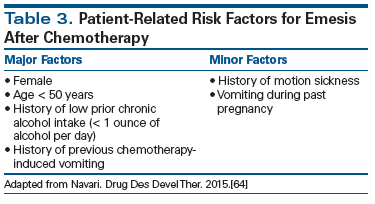
All major CINV guidelines categorize carboplatin as MEC. However, the emetogenic potential of carboplatin is likely underestimated; multiple studies in patients receiving carboplatin-based regimens report emesis rates in the range of 82% to 84% (by comparison, cisplatin-associated emesis rates are 91% to 97%), which is at the high end of emetogenic potential for MEC.[44] Additional studies evaluating the benefit of a three-drug regimen-with addition of an NK1 RA to a 5-HT3 RA and dexamethasone as CINV prophylaxis for patients receiving carboplatin-based chemotherapy-have shown incremental benefit with addition of NK1 RA in the range of 10% to 15%.[45-47]
A recent phase III study (n = 1,369) evaluated the addition of rolapitant to granisetron and dexamethasone as antiemetic treatment for patients receiving MEC and AC chemotherapy (65% of patients in the MEC group received carboplatin).[20] CR rates during the acute, delayed, and overall phases were statistically significant in favor of this three-drug regimen in the MEC group, demonstrating the benefit of more aggressive CINV prophylaxis for carboplatin-based regimens. Based on the available clinical data, NCCN and MASCC/ESMO have broadened their recommendations for use of an NK1 RA in combination with a 5-HT3 RA and dexamethasone for patients receiving carboplatin.
Nausea as a Pathophysiologic Entity Distinct From Vomiting
Usually in routine clinical practice, nausea and vomiting are considered and managed similarly. However, the mechanisms underlying nausea are complex, and the neural pathways are poorly understood. Nausea has been reported by many patients undergoing chemotherapy to be more distressing than emesis, as it can be disabling and long-lasting. Due to the subjective nature of nausea and a lack of a clinically useful biomarker, several validated questionnaires have been developed to identify the impact of nausea on quality of life, along with assessment of the efficacy of various antiemetics in patients undergoing chemotherapy.
Two commonly used patient-reported questionnaires include the Functional Living Index for Emesis (FLIE) and the MASCC Antiemesis Tool (MAT). MAT is an eight-item scale that is shorter and more convenient for patients, and includes a 24-hour recall period. FLIE is designed to assess the impact of acute and delayed CINV on quality of life and is validated for a 5-day recall period.
Guideline committees have recommended standardized approaches to the assessment of nausea across trials, in order to compare the efficacy of specific interventions in controlling nausea. Also, studies that include nausea as an outcome should include patient-reported measures of nausea, consistent with FDA recommendations.[3,48]
Future studies directed towards better understanding of the underlying pathophysiology of nausea and evaluation of potential clinically useful biomarkers for objective measurement of nausea might further improve control of chemotherapy-induced nausea.
The Role of Patient-Related Risk Factors
Despite recent advances in the prevention of CINV, control remains suboptimal.[49] Current guidelines for CINV prophylaxis are based solely on the emetogenic potential of chemotherapy. However, there are well-established patient-related factors that influence the risk for CINV; these include young age, female gender, history of low alcohol intake, and emesis during a previous pregnancy or course of chemotherapy (Table 3).[1,46,50] A comprehensive approach with incorporation of these factors into the development of antiemesis recommendations for individual patients appears reasonable. Particularly for patients receiving MEC, the addition of a third agent (eg, an NK1 RA) may improve CINV control. However, prospective studies are required to demonstrate whether such a model has a significant enough impact on CINV control to warrant a change in the current practice guidelines. A recent study by Clemons et al demonstrated that antiemetic prophylaxis based on a patient-related risk model led to improved control of CINV.[51] However, this study was limited by control arm patients’ low compliance with antiemetic guidelines.
Radiation Therapy–Induced Nausea and Vomiting
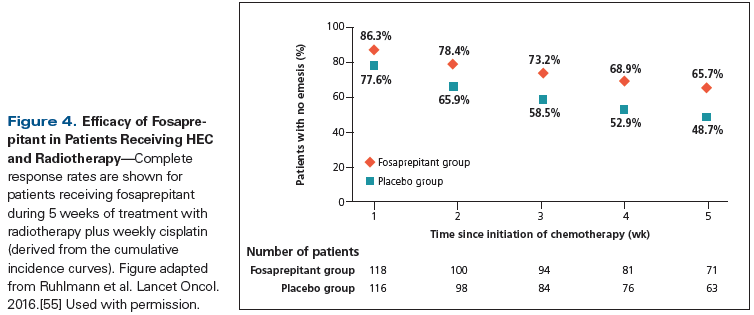
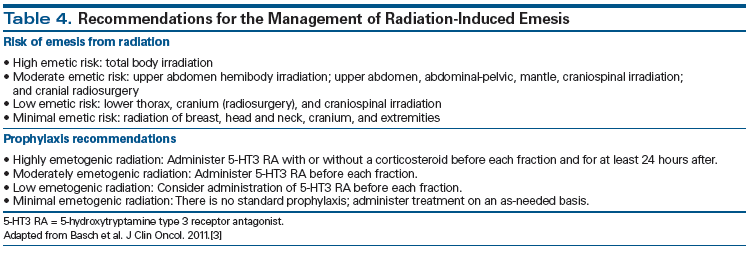
The importance of radiation therapy (RT)-induced nausea and vomiting with or without chemotherapy has been acknowledged by antiemetic guidelines, and is broadly divided into four risk categories: high risk of emesis (> 90%), moderate risk (> 60% to 90%), low risk (30% to 60%), and minimal risk (< 30%) (Table 4).[3,52] The combination of RT with chemotherapy and the site of delivery of RT are two important factors guiding prophylactic antiemetic recommendations. For concurrent chemoradiation, the risk of therapy-related nausea and vomiting is primarily dictated by the emetogenic potential of the chemotherapy regimen. Recent studies have suggested that 5-HT3 RAs are the preferred agents for preventing RT-induced vomiting,[53] and the addition of oral dexamethasone (4 mg daily) to 5-HT3 RAs confers additional modest efficacy against nausea and vomiting.[54] Management of breakthrough nausea and vomiting is similar to the approaches used for CINV, and the addition of rescue medication from a different class of drug is typically recommended, with preference given to use of a 5-HT3 RA if it is not administered as primary prophylaxis. A recent phase III trial compared triplet therapy with palonosetron, fosaprepitant, and dexamethasone vs a doublet regimen of palonosetron and dexamethasone in patients with cervical cancer treated with weekly cisplatin and pelvic RT. Over the entire course of treatment, emesis control was superior for patients who received the three-drug regimen (Figure 4), establishing a potential new standard of care in this setting.[55]
Patient Adherence to Antiemetic Guidelines
The best management approach for patients at risk of CINV is prevention. However, despite the recent availability of better medications for prophylaxis, about 20% to 25% of patients still do not achieve complete protection from CINV.[49] The observation that outcomes of clinical trials of potential agents for CINV prophylaxis are superior to results achieved in clinical practice suggests that adherence to guidelines is fundamental in controlling CINV.[56] Reports in the current medical literature indicate that adherence to antiemetic regimens for CINV prophylaxis is limited by both patient-related and healthcare system–related factors. Polypharmacy, drug-drug interactions, and the perception that CINV severity is related to an alteration in treatment may cause patients to under-report CINV events. Also, relying on the patient’s ability to recall CINV symptoms on a follow-up visit is not an accurate way to assess efficacy and adherence.[57] Quality patient education with improved self-reporting tools for feedback can address this issue.[58]
One European study evaluating the benefit of regular patient education by a pharmacist (with medication counseling) before and during chemotherapy demonstrated a significantly decreased frequency of CINV in the intervention group.[59] Healthcare professionals also fail to accurately predict the possibility of CINV, particularly during the delayed phase; physician knowledge of and agreement with guidelines, as well as familiarity with the patient’s medications, play an important role in this setting.[60,61] One study that combined an education session for healthcare providers, a risk-assessment tool with guideline-based order sets, and a feedback system increased physician adherence to the guideline from 58% to 90%, along with reduction in frequency of patient-reported CINV.[62] Other factors that may limit adherence to CINV management guidelines include cost of, and access to, the appropriate prophylactic medications.[63]
Conclusion and Future Directions
There has been significant improvement in the prevention of CINV in the last 5 years, with the introduction of new agents and appropriate reclassification of the emetogenic risk of certain chemotherapeutic agents. However, despite new and potentially more convenient prophylactic choices, control of CINV is still suboptimal. Further testing of currently approved agents in head-to-head studies, or the use of an additional agent in highly emetogenic or refractory cases, is warranted. Additional modeling of patient-related factors may lead to more precision in predicting the risk of CINV, particularly in the MEC category of chemotherapies. To ensure that best practices are followed in preventing CINV requires a multidisciplinary, patient-centered approach that includes appropriate educational initiatives for patients and healthcare professionals. Establishing a robust feedback system to assess the efficacy of, and adherence to, specific medications for prophylaxis against CINV could increase real-world effectiveness. Finally, development of additional agents utilizing the increased body of knowledge about the role of specific neurotransmitters in CINV-particularly as they relate to the persistent problem of delayed nausea-could further reduce the residual burden of CINV. It is gratifying to see that this common side effect of chemotherapy continues to occupy the attention of researchers, clinicians, and patients, with a common goal of eradicating it completely.
Financial Disclosure: Dr. Schwartzberg serves as a consultant to Eisai, Helsinn, Merck, and Tesaro; and he receives research funding from Helsinn. Dr. Nasir has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: antiemesis. Version 2.2016. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed June 19, 2016.
2. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232-v243.
3. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-98.
4. Kris MG, Gralla RJ, Clark RA, et al. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol. 1985;3:1279-84.
5. Navari RM. Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting-two new agents. J Support Oncol. 2003;1:89-103.
6. Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482-94.
7. Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol. 2014;722:26-37.
8. Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol. 2016;34:381-6.
9. Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) antiemetic guideline 2016. http://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_guidelines_english_2016_v.1.1.pdf. Accessed June 6, 2016.
10. Saito R, Takano Y, Kamiya HO. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci. 2003;91:87.
11. Spinelli T, Calcagnile S, Giuliano C, et al. Netupitant PET imaging and ADME studies in humans. J Clin Pharmacol. 2014;54:97-108.
12. Lanzarotti C, Rossi G. Effect of netupitant, a highly selective NK1 receptor antagonist, on the pharmacokinetics of midazolam, erythromycin, and dexamethasone. Support Care Cancer. 2013;21:2783-91.
13. Giuliano C, Lovati E, Funk C, et al. In vitro drug-drug interaction studies with the antiemetic drug netupitant and its major metabolites (M1 and M2), involving main human cytochrome P450 isoenzymes. Ann Oncol. 2012;23:ix499–ix527.
14. Stathis M, Pietra C, Rojas C, et al. Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol. 2012;689:25-30.
15. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-6.
16. Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328-33.
17. Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25:1333-9.
18. Poma A, Christensen J, Davis J, et al. Phase 1 positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J Clin Oncol. 2014;32(suppl):abstr e20690.
19. Poma A, Christensen J, Pertikis H, et al. Rolapitant and its major metabolite do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Presented at the Multinational Association of Supportive Care in Cancer 2013 annual meeting; Berlin, Germany; June 27–29, 2013. Abstr 441.
20. Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. 2015;16:1071-8.
21. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-89.
22. Perez EA, Hesketh P, Sandbach J, et al. Comparison of single-dose oral granisetron versus intravenous ondansetron in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy: a multicenter, double-blind, randomized parallel study. J Clin Oncol. 1998;16:754-60.
23. Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005;23:1289-94.
24. Stoltz R, Cyong JC, Shah A, et al. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol. 2004;44:520-31.
25. Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT(3) and tachykinin NK(1) receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2012;684:1-7.
26. Gralla R, Lichinitser M, Van Der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570-7.
27. Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98:2473-82.
28. Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441-9.
29. Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115-24.
30. Aogi K, Sakai H, Yoshizawa H, et al. A phase III open-label study to assess safety and efficacy of palonosetron for preventing chemotherapy-induced nausea and vomiting (CINV) in repeated cycles of emetogenic chemotherapy. Support Care Cancer. 2012;20:1507-14.
31. Lorusso V, Giampaglia M, Petrucelli L, et al. Antiemetic efficacy of single-dose palonosetron and dexamethasone in patients receiving multiple cycles of multiple day-based chemotherapy. Support Care Cancer. 2012;20:3241-6.
32. Schwartzberg L, Barbour S, Morrow G, et al. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron and granisetron in the prevention of chemotherapy induced nausea and vomiting (CINV). Support Care Cancer. 2014;22:469-77.
33. Boccia R, Grunberg S, Franco-Gonzales E, et al. Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trial. Support Care Cancer. 2013;21:1453-60.
34. Karthaus M, Tibor C, Lorusso V, et al. Efficacy and safety of oral palonosetron compared with IV palonosetron administered with dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin-based highly emetogenic chemotherapy (HEC). Support Care Cancer. 2015;23:2917-23.
35. Broder MS, Faria C, Powers A, et al. The impact of 5-HT3RA use on cost and utilization in patients with chemotherapy-induced nausea and vomiting: systematic review of the literature. Am Health Drug Benefits. 2014;7:171-82.
36. Ottoboni T, Gelder M, O’Boyle E. BiochronomerTM technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
37. Gabrail N, Yanagihara R, SpaczyÅski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase 2 trials. Cancer Manag Res. 2015;7:83-92.
38. Raftopoulos H, Cooper W, O’Boyle E, et al. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-32.
39. Schnadig I, Agajanian R, Dakhil C, et al. APF530 (granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-81.
40. Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2013;21:1655-63.
41. Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87-96.
42. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9:188-95.
43. Eli Lilly and Company. Zyprexa prescribing information. Revised July 23, 2015. http://pi.lilly.com/us/zyprexa-pi.pdf. Accessed June 8, 2016.
44. Hannigan EV, Green S, Alberts DS, et al. Results of a Southwest Oncology Group phase III trial of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide in advanced ovarian cancer. Oncology. 1993;50(suppl 2):2-9.
45. Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol. 2015;26:1081-90.
46. Gralla R, Jordan K, Rapoport B, et al. Assessing the magnitude of antiemetic benefit with the addition of the NK1 receptor antagonist (NK1) aprepitant for all platinum agents: analysis of 1,872 patients in prospective randomized clinical phase III trials (RCTs). J Clin Oncol. 2010;28(suppl):abstr 9057.
47. Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. 2010;18:423-31.
48. US Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/.../Guidances/UCM193282.pdf. Accessed June 6, 2016.
49. Schwartzberg L. Addressing the value of novel therapies in chemotherapy-induced nausea and vomiting. Expert Rev Pharmacoecon Outcomes Res. 2014;14:825-34.
50. Grunberg SM, Osoba D, Hesketh PJ, et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity-an update. Support Care Cancer. 2005;13:804.
51. Clemons M, Bouganim N, Smith S, et al. Risk model–guided antiemetic prophylaxis vs physician’s choice in patients receiving chemotherapy for early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2016;2:225-31.
52. Feyer PC, Maranzano E, Molassiotis A, et al. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer. 2011;19(suppl 1):S5-S14.
53. Salvo N, Doble B, Khan L, et al. Prophylaxis of radiation-induced nausea and vomiting using 5-hydroxytryptamine-3 serotonin receptor antagonists: a systematic review of randomized trials. Int J Radiat Oncol Biol Phys. 2012;82:408-17.
54. Wong RK, Paul N, Ding K, et al. 5-hydroxytryptamine-3 receptor antagonist with or without short-course dexamethasone in the prophylaxis of radiation induced emesis: a placebo-controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19). J Clin Oncol. 2006;24:3458-64.
55. Ruhlmann CH, Cristensen TB, Dohn LH, et al. Efficacy and safety of fosaprepitant for the prevention of nausea and emesis during 5 weeks of chemoradiotherapy for cervical cancer (the GAND-emesis study): a multinational, randomised, placebo-controlled, double-blind, phase 3 trial. Lancet Oncol. 2016;17:509-18.
56. Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent anti-emetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol. 2012;23:1986-92.
57. Jordan K, Gralla R, Jahn F, et al. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197-202.
58. Navari RM, Aapro MS. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-67.
59. Liekweg A, Westfeld M, Braun M, et al. Pharmaceutical care for patients with breast and ovarian cancer. Support Care Cancer. 2012;20:2669-77.
60. Grunberg SM. Obstacles to implementation of antiemetic guidelines. J Natl Compr Canc Netw. 2009;7:601-5.
61. Kaiser R. Antiemetic guidelines: are they being used? Lancet Oncol. 2005;6:622-5.
62. Affronti ML, Schneider SM, Herndon JE II, et al. Adherence to antiemetic guidelines in patients with malignant glioma: translating evidence into practice. Support Care Cancer. 2014;22:1897-905.
63. Viale PH, Grande C, Moore S. Efficacy and cost: avoiding under treatment of chemotherapy-induced nausea and vomiting. Clin J Oncol Nurs. 2012;16:E133-E141.
64. Navari RM. Profile of netupitant/palonosetron (NEPA) fixed-dose combination and its potential in the treatment of chemotherapy-induced nausea and vomiting. Drug Des Devel Ther. 2015;9:155-61.
65. Hesketh PJ, Aapro M, Jordan K, et al. A review of NEPA, a novel fixed antiemetic combination with the potential for enhancing guideline adherence and improving control of chemotherapy-induced nausea and vomiting. BioMed Res Int. 2015;2015:651879.