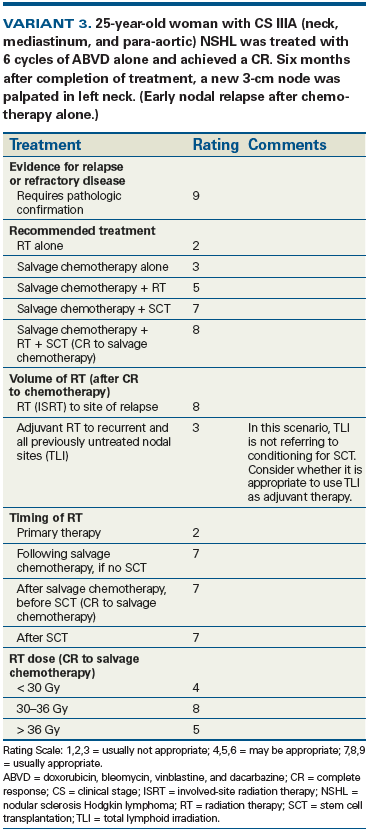
This topic addresses the management of recurrent Hodgkin lymphoma. While autologous stem cell transplantation may be appropriate for select cases of recurrent disease following comprehensive combined-modality therapy, other options exist for patients treated with lower-dose therapy for early-stage disease. Additionally, innovative targeted therapies provide newer salvage options to consider. The American College of Radiology Appropriateness Criteria® are evidence-based guidelines for specific clinical conditions that are reviewed annually by a multidisciplinary expert panel. The guideline development and revision include an extensive analysis of current medical literature from peer-reviewed journals and the application of well-established methodologies (RAND/UCLA Appropriateness Method and Grading of Recommendations Assessment, Development, and Evaluation, or GRADE) to rate the appropriateness of imaging and treatment procedures for specific clinical scenarios. In those instances where evidence is lacking or equivocal, expert opinion may supplement the available evidence to recommend imaging or treatment. By combining the most recent medical literature and expert opinion, this revised guideline can aid clinicians in the complex decision-making associated with the management of recurrent Hodgkin lymphoma.
Summary of Literature Review
Introduction/Background
Classical Hodgkin lymphoma is a highly curable cancer, even in advanced stages. Although radiation therapy (RT) alone improved disease-free survival (DFS) for many years, the management of classical Hodgkin lymphoma has changed dramatically over the past 2 decades with the use of highly effective systemic therapies and the subsequent reduction in the use of radiation.[1] Even with combined-modality therapy (CMT), rates of relapse can vary from 5% for early-stage disease to 35% for more advanced stages.[2,3] Approximately 10% of patients will have disease that is refractory to initial therapy.[4] Even in the setting of relapsed or refractory disease, classical Hodgkin lymphoma remains salvageable. The standard of care for relapsed/refractory disease is either conventional chemotherapy or high-dose chemotherapy with autologous stem cell transplantation (HDCT + ASCT). The role of RT in relapsed/refractory disease remains controversial and is reviewed in these guidelines.
Definitions and Determination of Relapsed/Refractory Disease
Relapse or recurrence can be defined as the reappearance of disease after initial therapy and complete response (CR) in the site of prior disease and/or in new sites. Progression refers to evidence of increasing disease after achievement of stable disease, partial remission (PR), or CR, whereas refractory disease is a failure to achieve either a CR or PR and may represent a more significant degree of radiation or drug resistance.[5,6]
Current National Comprehensive Cancer Network guidelines recommend biopsy to document relapse, progression, or refractory disease.[7] Until recently, guidelines as to how to document progression of disease in the setting of incomplete remission remained unclear.[8] Therefore, it is uncertain whether in practice biopsies are routinely performed according to this standard. However, biologic confirmation of disease is recommended. A biopsy may also be warranted in patients whose disease is refractory to therapy to confirm the initial diagnosis of classical Hodgkin lymphoma.
The majority of relapses following a CR in patients treated for classical Hodgkin lymphoma occur within 3 years of therapy, so routine surveillance by clinical examination is an essential component of a survivorship plan (see the ACR Appropriateness Criteria® Follow-up of Hodgkin Lymphoma[9]). The use of routine imaging after a CR is being challenged by recent studies,[10] so decisions regarding use can be made on an individual basis. A clear plan for surveillance is crucial, as timing of relapse has important prognostic significance and may impact treatment options.[3] Early relapse (< 12 months) is a poor prognostic factor and warrants more aggressive therapy. Other prognostic factors include localized vs disseminated disease and disease that has relapsed in previously irradiated areas.[11]
Management of Relapse Following Chemotherapy or CMT
HDCT + ASCT is the standard of care for relapsed/refractory classical Hodgkin lymphoma and can induce durable remissions in > 50% of patients.[12] No randomized trials have compared the effectiveness of salvage regimens for classical Hodgkin lymphoma, so selecting the appropriate regimen may be a challenge when considering both efficacy and toxicities. Because the goal of salvage chemotherapy is to achieve a second CR, usually a regimen different from that used for the initial course of therapy is administered.[13]
Some institutions favor platinum-based multidrug regimens, such as ICE (ifosfamide, carboplatin, and etoposide) or DHAP (dexamethasone, cytarabine, and cisplatin).[14,15] More recently, gemcitabine-based regimens[16,17] and the use of bendamustine[18] have been explored; these regimens are effective and well tolerated even in heavily pretreated patients. Gemcitabine-based regimens have been used both as primary salvage and for secondary salvage after ASCT.[16,18,19] Few studies have been done comparing the efficacy of different multidrug regimens. A small prospective study of 44 patients compared GDP (gemcitabine, dexamethasone, and cisplatin) with ESHAP (etoposide, methylprednisolone, cisplatin, and cytarabine); no difference was found in the response rate for relapsed/refractory Hodgkin lymphoma.[20]
Patients with relapsed or refractory disease after initial salvage have a median survival of < 3 years with standard therapies.[21] Newer biologics and targeted therapies, such as brentuximab vedotin and nivolumab, may further improve survival. Brentuximab vedotin (SGN-35) is a CD30-directed antibody linked to monomethyl auristatin E, an antitubulin agent.[21] In a phase I trial, this potent antibody-drug conjugate was able to induce a CR or PR in 17 of 45 patients with relapsed or refractory disease who had been treated with multiple prior therapies.[22] The other 19 cases had stable disease after treatment. In a more recent multicenter, prospective, phase II study, the use of brentuximab enabled 86% of patients with relapsed/refractory Hodgkin lymphoma to proceed to ASCT.[23] The conjugate has also been shown to be effective in patients with relapsed/refractory disease after prior autologous or allogeneic stem cell transplantation, with overall response rates of 75% and 50%, respectively.[24,25] Nivolumab is a programmed death 1 (PD-1)–blocking antibody that has considerable activity even in patients heavily pretreated for relapsed/refractory Hodgkin lymphoma who had previously failed brentuximab vedotin.[26] Other targeted agents currently in development may also impact outcomes in the setting of relapsed/refractory classical Hodgkin lymphoma.[27]
Role of RT in Hodgkin Lymphoma Salvage Programs
Poen et al[28] reported the results of a prospective trial evaluating the efficacy of involved-field radiation therapy (IFRT) as part of the salvage regimen in patients selected for ASCT. Of 100 patients with relapsed/refractory classical Hodgkin lymphoma planning to proceed to ASCT, 24 received IFRT either before (n = 18) or after (n = 6) transplant. In patients with relapsed stage I–III classical Hodgkin lymphoma, the use of IFRT was associated with an improved 3-year freedom from relapse (100% vs 67%; P = .04) and a trend toward improved overall survival (OS) (85% vs 60%; P = .16).
Two additional contemporary retrospective studies also showed that IFRT following HDCT + ASCT improves local control and survival in refractory disease, particularly in patients who have bulky disease at the time of relapse.[29,30] In one analysis, IFRT conferred benefits with respect to both 3-year OS (69.6% vs 40%) and disease-specific survival (82.1% vs 57.6%).[29] The timing of when to administer IFRT, either before or after ASCT, has been debated. When radiation is administered prior to ASCT, patients are more likely to receive the radiation as planned. Any marrow that may be in the field will be replaced once the patient’s transfused stem cells engraft, and there is a greater chance that the patient will enter ASCT with no residual disease. Some may prefer delivering radiation after ASCT to minimize the interval between the last dose of chemotherapy and ASCT, although several centers are accelerating the course of radiation to accommodate a quicker transition to ASCT. There is also a concern that radiation before ASCT may delay treatment plans because of increased toxicity; however, in the modern era, involved-site radiation therapy (ISRT) is the preferred method used in the management of both Hodgkin lymphoma and non-Hodgkin lymphoma,[31] and the smaller fields may reduce risk of acute treatment-related toxicities.
Total lymphoid irradiation (TLI) can be utilized as part of conditioning regimens prior to ASCT. A recent study evaluated the use of positron emission tomography (PET)/computed tomography (CT) to predict outcomes of 51 patients treated for relapsed/refractory disease with TLI followed by HDCT + ASCT.[32] The 10-year progression-free survival (PFS) and OS rates were 56% and 54%, respectively. There are several ongoing clinical trials utilizing TLI as part of nonmyeloablative, reduced-intensity conditioning regimens for several hematologic disorders, including Hodgkin lymphoma (www.ClinicalTrials.gov). Some institutions have included total body irradiation (TBI) as part of the pretransplant conditioning regimen.[28,33,34] A feasibility study of tandem ASCT for relapsed/refractory classical Hodgkin lymphoma compared myeloablative regimens containing either busulfan or 12 Gy of TBI for patients making it to the second transplant.[33] Of the 43 patients enrolled, 32 received the second ASCT, with half receiving TBI. An additional 20 Gy of IFRT was included for 5 of these patients. In this particular study, the use of TBI did not increase risk of acute or late toxicities when compared with the group receiving chemotherapy alone.
Role of RT in Advanced-Stage Chemorefractory Hodgkin Lymphoma
Limited data are available on the role of consolidative RT in the setting of residual fluorine-18–2-fluoro-2-deoxy-D-glucose (FDG) avidity following combination chemotherapy for patients with advanced classical Hodgkin lymphoma. Sher et al[35] performed a retrospective analysis of 73 patients who received consolidative IFRT following systemic therapy to evaluate the prognostic significance of residual FDG avidity. The majority of patients (n = 60) were PET-negative at the end of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) therapy; the actuarial 2-year failure-free survival rate was 97% for this group, with 100% salvage for patients who relapsed. The 2-year failure-free survival rate was 69% for the PET-positive group with IFRT doses of ≥ 30 Gy, generating the hypothesis that the addition of RT may successfully sterilize local residual disease, thereby precluding the need to proceed immediately to salvage therapy in this setting.
Summary of Recommendations
- Hodgkin lymphoma is a highly curable disease, even in the relapsed setting.
- Although the standard of care for relapsed disease remains salvage therapy followed by ASCT, other alternatives exist in the modern era.
- Biologics and targeted agents such as brentuximab vedotin and nivolumab offer alternative systemic therapies that may get patients to ASCT faster and with less toxicity.
- The role of RT in the relapsed setting remains controversial, but prognostic factors such as timing and extent of relapse may help guide clinical decision making.
- In selected patients with small isolated relapses that occur > 3 years after initial presentation, consideration may also be given to a course of RT alone or CMT without transplant.
- RT is particularly indicated as part of CMT for patients with local relapse after treatment with chemotherapy alone or for relapses outside of the original site of disease; in this setting, ASCT may be deferred.
- ASCT should be considered for all patients with early relapsed or progressive disease while on therapy. If there is a PR to salvage chemotherapy, RT may be given pretransplant to achieve a CR.
- If a patient has a CR and the timing of RT is not dictated by a clinical trial, consolidative RT after ASCT allows the patient to proceed with therapy that is proven to improve OS.
- Modern radiation techniques, smaller treatment field, and lower overall doses help improve the therapeutic ratio of RT.
- Decisions regarding the best options in the relapsed setting should be made in the multidisciplinary setting.
In the European Organisation for Research and Treatment of Cancer (EORTC) 20884 trial, patients with stage III/IV classical Hodgkin lymphoma were treated with 6 to 8 cycles of MOPP-ABV (mechlorethamine, vincristine, procarbazine, and prednisone with doxorubicin, bleomycin, and vinblastine); patients who achieved a PR received 30-Gy IFRT.[36] The freedom from treatment failure (FFTF) and OS for this group were similar to patients who had achieved a CR in this study (5-year event-free survival, 79%; 5-year OS, 87%), suggesting that IFRT following a PR to systemic therapy improves outcomes.
Similarly, the German Hodgkin Study Group (GHSG) HD15 trial for patients with advanced-stage classical Hodgkin lymphoma administered 30-Gy IFRT to patients with PET-positive disease that measured ≥ 2.5 cm on CT scan after BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone).[37] Of 311 patients who met criteria for enrollment, PET was positive in 66 patients; RT was recommended for 63. The PFS rate at 18 months was 96% for PET-negative patients and 85% for PET-positive patients, indicating that IFRT for patients with persistently FDG-avid disease can successfully sterilize residual disease and may be a viable alternative to HDCT + ASCT.
Salvage Without Stem Cell Transplantation
Although HDCT + ASCT is the standard of care for relapsed/refractory classical Hodgkin lymphoma, several scenarios exist where alternative treatment strategies could be considered. These include patients with late relapse (> 1 year), patients who received limited frontline therapies such as RT or chemotherapy alone, or patients with early-stage disease treated with reduced-intensity therapy (eg, 2 cycles of ABVD followed by 20-Gy RT).
Relapse following RT alone
Historical series suggest that approximately 20% to 35% of patients with early-stage Hodgkin lymphoma treated with RT alone will relapse.[38,39] Yet there were many variables across studies, including staging with or without laparotomy, inclusion of patients with different prognostic factors, and differences in radiation field size, which may have impacted DFS and therefore make comparing studies a challenge. Ng et al[40] described outcomes of a single-institution prospective trial evaluating the use of mantle irradiation alone in patients with early-stage classical Hodgkin lymphoma. Patients with stage I–IIA classical Hodgkin lymphoma were enrolled in the study; patients with B symptoms, bulky disease, or subcarinal or hilar involvement were excluded. Mantle radiation with doses ranging from 30 to 40 Gy was delivered from 1988 to 2000 in a cohort of patients with a median age of 30 years. Of the 87 patients enrolled, 13 (15%) relapsed, with most sites of relapse outside of the original treatment field. Half of the patients were salvaged with ABVD alone; the other half had CMT with IFRT. Only 1 patient experienced a second relapse and underwent salvage with HDCT followed by stem cell transplantation (SCT).[40]
An earlier retrospective study from MD Anderson Cancer Center enrolling patients beginning in 1967 included patients with unfavorable disease, including bulky tumor, > 4 sites of disease, and/or B symptoms.[41] The 10-year PFS rate following mantle irradiation for this cohort that included patients with higher-risk disease was 75.3%. However, 10- and 20-year actuarial OS rates were 87.6% and 65.3%, respectively, confirming highly successful salvage rates after treatment with radiation alone.
Although the use of RT as a primary treatment of classical Hodgkin lymphoma is no longer the standard of care, for the small cohort of patients previously treated with RT alone, it is still important to bear in mind that chemotherapy alone or with RT may be sufficient for salvage. However, limited data exist to provide definitive treatment recommendations.
Relapse following reduced-intensity therapy for early-stage disease
Reduced-intensity therapy with 2 cycles of ABVD followed by 20-Gy IFRT for patients with favorable early-stage classical Hodgkin lymphoma provides favorable outcomes, with a FFTF rate of 91.1% at 5 years.[2] Since relapse in this very favorable group is uncommon, decisions must be made regarding the appropriate salvage therapies. Little data exist regarding the best salvage options in this setting.
The GHSG assessed results of salvage therapy in 42 patients out of 1,129 who were enrolled in 3 different prospective trials evaluating the use of 2 cycles of ABVD followed by IFRT.[11] About 50% of these patients experienced an infield relapse, and a variety of salvage regimens were employed, including chemotherapy, HDCT + ASCT, and RT alone. In this small cohort, which had a median follow-up of 3 years, the OS rate was 67% following salvage therapy. Although this small report provides some insight into salvage following reduced-intensity therapy, additional prospective studies are needed to define the optimal salvage regimen in this cohort. In the meantime, extrapolation from these data regarding treatment of relapses with either chemotherapy or RT alone should help make informed decisions.
Relapse following chemotherapy alone
In the modern era, most patients diagnosed with Hodgkin lymphoma receive chemotherapy as part of initial therapy, either with or without consolidative RT. Treatment options at the time of relapse will vary based on a variety of prognostic features, the most significant of which are the presence of refractory disease and early and advanced relapses.[42]
Despite evidence suggesting that the addition of RT to systemic therapy improves tumor control and OS in patients with early-stage classical Hodgkin lymphoma,[34,43] efforts are still underway to validate the use of chemotherapy alone as a means to decrease late treatment-related toxicities. One retrospective study reports the results of patients with early-stage classical Hodgkin lymphoma who were treated with chemotherapy alone from 1992 to 2008.[44] Bulky disease was excluded and eligible patients received 6 cycles of ABVD. All 71 of the patients included in the study achieved a CR. At a median follow-up of 60 months, there were 6 relapses (8%), all at the site of presenting disease. Five of the relapses occurred within the first 2 years after a CR, and salvage consisted of CMT followed by ASCT.
It bears mentioning that salvage RT alone may be sufficient in these patients with local recurrence. Josting et al[45] reported the results of salvage RT for patients enrolled in GHSG trials from 1988 to 1999 who relapsed; most had been treated with COPP (cyclophosphamide, vincristine, procarbazine, and prednisone)/ABVD–like regimens. Salvage RT was used to treat patients at the time of initial relapse; the treatment field varied but mantle or IFRT was most often applied. Of the 100 patients, 77 achieved a CR. Sixty-eight of these patients (88%) had stage I/II disease.[45] In this largest series evaluating use of salvage RT, the 5-year FFTF and OS rates were 28% and 51%, respectively, highlighting the need for appropriate patient selection.
Likewise, the management of patients who initially received chemotherapy alone for early-stage disease and then relapsed locally must be carefully considered when outlining a treatment plan. Although ASCT has been shown to improve OS in relapsed classical Hodgkin lymphoma, it may not be necessary in this particular cohort of patients.[42] For each case, the long-term toxicity of additional chemotherapy must be weighed against that of modern RT (see Variants 1–6).
The American College of Radiology seeks and encourages collaboration with other organizations on the development of the ACR Appropriateness Criteria® through society representation on expert panels. Participation by representatives from collaborating societies on the expert panel does not necessarily imply individual or society endorsement of the final document.
Financial Disclosure:Dr. Flowers is a consultant for OptumRX. Dr. Younes receives research support from Curis, Johnson & Johnson, and Novartis; and receives honoraria from Bayer, Bristol Myers-Squibb, Celgene, Incyte, Janssen, Sanofi, Seattle Genetics, and Takeda Millennium.
Copyright © 2016 American College of Radiology. Reprinted with permission of the American College of Radiology.
Supporting Documents: For additional information on the Appropriateness Criteria® methodology and other supporting documents, go to www.acr.org/ac.
References:
1. Gobbi PG, Ferreri AJ, Ponzoni M, Levis A. Hodgkin lymphoma. Crit Rev Oncol Hematol. 2013;85:216-37.
2. Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640-52.
3. Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002;20:221-30.
4. Ferme C, Mounier N, Divine M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol. 2002;20:467-75.
5. Canioni D, Deau-Fischer B, Taupin P, et al. Prognostic significance of new immunohistochemical markers in refractory classical Hodgkin lymphoma: a study of 59 cases. PLoS One. 2009;4:e6341.
6. Josting A, Rueffer U, Franklin J, et al. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96:1280-6.
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Hodgkin lymphoma. Version 2.2015. http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. Accessed October 25, 2016.
8. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-68.
9. Ha CS, Hodgson DC, Advani R, et al. ACR Appropriateness Criteria Follow-up of Hodgkin lymphoma. J Am Coll Radiol. 2014;11:1026-33.
10. Gandikota N, Hartridge-Lambert S, Migliacci JC, et al. Very low utility of surveillance imaging in early-stage classic Hodgkin lymphoma treated with a combination of doxorubicin, bleomycin, vinblastine, and dacarbazine and radiation therapy. Cancer. 2015;121:1985-92.
11. Sieniawski M, Franklin J, Nogova L, et al. Outcome of patients experiencing progression or relapse after primary treatment with two cycles of chemotherapy and radiotherapy for early-stage favorable Hodgkin’s lymphoma. J Clin Oncol. 2007;25:2000-5.
12. Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1065-72.
13. Mendler JH, Friedberg JW. Salvage therapy in Hodgkin’s lymphoma. Oncologist. 2009;14:425-32.
14. Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13:1628-35.
15. Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616-23.
16. Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071-9.
17. William BM, Loberiza FR Jr, Whalen V, et al. Impact of conditioning regimen on outcome of 2-year disease-free survivors of autologous stem cell transplantation for Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2013;13:417-23.
18. Kuruvilla J, Nagy T, Pintilie M, et al. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106:353-60.
19. Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica. 2007;92:35-41.
20. Ramzi M, Rezvani A, Dehghani M. GDP versus ESHAP regimen in relapsed and/or refractory Hodgkin lymphoma: a comparison study. Int J Hematol Oncol Stem Cell Res. 2015;9:10-4.
21. Horning SJ, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: an international effort. Ann Oncol. 2008;19:iv120-iv121.
22. Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16:888-97.
23. Chen R, Palmer JM, Martin P, et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21:2136-40.
24. Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812-21.
25. Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183-9.
26. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311-9.
27. Gopal AK, Ramchandren R, O’Connor OA, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120:560-8.
28. Poen JC, Hoppe RT, Horning SJ. High-dose therapy and autologous bone marrow transplantation for relapsed/refractory Hodgkin’s disease: the impact of involved field radiotherapy on patterns of failure and survival. Int J Radiat Oncol Biol Phys. 1996;36:3-12.
29. Biswas T, Culakova E, Friedberg JW, et al. Involved field radiation therapy following high dose chemotherapy and autologous stem cell transplant benefits local control and survival in refractory or recurrent Hodgkin lymphoma. Radiother Oncol. 2012;103:367-72.
30. Wendland MM, Asch JD, Pulsipher MA, et al. The impact of involved field radiation therapy for patients receiving high-dose chemotherapy followed by hematopoietic progenitor cell transplant for the treatment of relapsed or refractory Hodgkin disease. Am J Clin Oncol. 2006;29:189-95.
31. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89:854-62.
32. Gentzler RD, Evens AM, Rademaker AW, et al. F-18 FDG-PET predicts outcomes for patients receiving total lymphoid irradiation and autologous blood stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Br J Haematol. 2014;165:793-800.
33. Brice P, Divine M, Simon D, et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin’s disease (HD). SFGM/GELA Study Group. Ann Oncol. 1999;10:1485-8.
34. Herbst C, Rehan FA, Skoetz N, et al. Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2011:CD007110.
35. Sher DJ, Mauch PM, Van Den Abbeele A, et al. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma: the importance of involved-field radiotherapy. Ann Oncol. 2009;20:1848-53.
36. Aleman BM, Raemaekers JM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348:2396-406.
37. Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791-9.
38. Horwich A, Specht L, Ashley S. Survival analysis of patients with clinical stages I or II Hodgkin’s disease who have relapsed after initial treatment with radiotherapy alone. Eur J Cancer. 1997;33:848-53.
39. Tubiana M, Henry-Amar M, Carde P, et al. Toward comprehensive management tailored to prognostic factors of patients with clinical stages I and II in Hodgkin’s disease. The EORTC Lymphoma Group controlled clinical trials: 1964-1987. Blood. 1989;73:47-56.
40. Ng AK, Li S, Neuberg D, et al. Long-term results of a prospective trial of mantle irradiation alone for early-stage Hodgkin’s disease. Ann Oncol. 2006;17:1693-7.
41. Liao Z, Ha CS, Vlachaki MT, et al. Mantle irradiation alone for pathologic stage I and II Hodgkin’s disease: long-term follow-up and patterns of failure. Int J Radiat Oncol Biol Phys. 2001;50:971-7.
42. Brice P. Managing relapsed and refractory Hodgkin lymphoma. Br J Haematol. 2008;141:3-13.
43. Laskar S, Gupta T, Vimal S, et al. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? J Clin Oncol. 2004;22:62-8.
44. Canellos GP, Abramson JS, Fisher DC, LaCasce AS. Treatment of favorable, limited-stage Hodgkin’s lymphoma with chemotherapy without consolidation by radiation therapy. J Clin Oncol. 2010;28:1611-5.
45. Josting A, Nogova L, Franklin J, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23:1522-9.