Secondary central nervous system (CNS) relapse in aggressive non-Hodgkin lymphoma (NHL) is a dismal diagnosis with poor outcomes. While prophylaxis against secondary CNS disease is recommended in patients with highly aggressive NHLs, such as Burkitt lymphoma, patients with diffuse large B-cell lymphoma (DLBCL) present a challenging clinical dilemma due to an inherently lower risk of CNS relapse. Current guidelines suggest that prophylaxis may benefit DLBCL patients at high risk for CNS relapse; however, it has been difficult to define which patients are truly at high risk. Many studies have attempted to clarify the issue, with conflicting results. Here we review current prognostic models, risk factors, and prophylaxis methods to provide a practical approach to preventing CNS relapse in patients with DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for approximately one-third of adult non-Hodgkin lymphomas (NHLs) diagnosed annually.[1] Secondary central nervous system (CNS) involvement is a complication that occurs in approximately 5% of patients with DLBCL. Most cases of secondary CNS involvement, often termed CNS relapse, occur within the first year after the diagnosis of systemic disease, and patients have a median survival of only a few months.[2-4] Examples of such dismal outcomes are demonstrated by the RICOVER-60 trial, in which the median survival after CNS relapse was just 2.5 months, and approximately one-third of the 58 patients who developed CNS relapse did so after achieving a complete remission.[4] In a 20-year follow-up analysis, the Southwest Oncology Group found that 25 patients experienced CNS relapse, all of which occurred within 2 years of diagnosis (median, 5.4 months), with a median survival of only 2.2 months after CNS relapse.[2] In contrast to highly aggressive NHLs such as Burkitt lymphoma and lymphoblastic lymphoma, where the rate of CNS relapse exceeds 30%, CNS relapse prophylaxis is not routinely incorporated into treatment for most patients with DLBCL because of the infrequent occurrence of this complication.[5] While prophylaxis may reduce the risk of CNS relapse in DLBCL patients, it also exposes them to the drug-related toxicities and procedural risks associated with systemic or intrathecal (IT) chemotherapy. Determining which patients may benefit the most from prophylaxis and which methods of prophylaxis are most beneficial remain the most prominent issues to be settled.
Defining High-Risk Patients
Prognostic models for CNS relapse
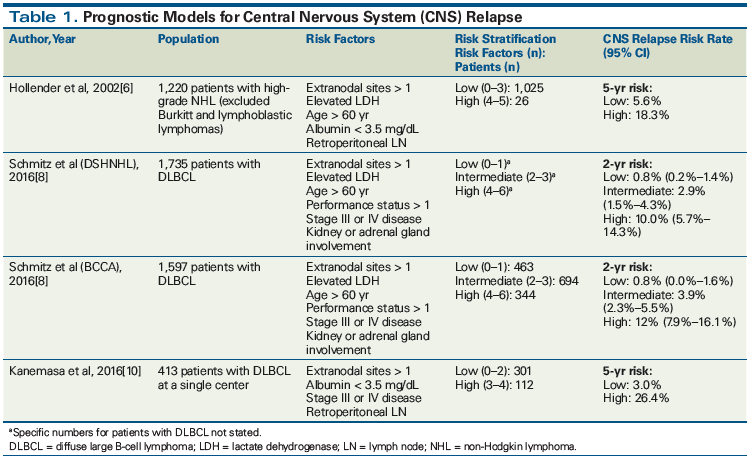
Defining which patients are high risk has proven to be a difficult task. Hollender et al developed a prognostic model consisting of extranodal sites > 1, albumin level < 3.5 mg/dL, age > 60 years, elevated lactate dehydrogenase (LDH) level, and retroperitoneal lymph node involvement, based on a multivariate analysis from their 2002 retrospective study.[6] Of 1,220 patients with high-grade NHL, 52 (4.3%) developed CNS involvement.[6] While not specifying the percentage of patients with DLBCL, patients classified as high risk comprised 12% of the cohort. Of those who subsequently developed CNS relapse, 54% were high risk. Further, high-risk patients had a CNS relapse rate of 25% compared with a relapse rate of 6% in low-risk patients. Notably, 141 patients (11.6%; most with extranodal involvement) received CNS prophylaxis; however, CNS relapse rates in these patients were not explicitly stated. The investigators recommend stratifying patients as low risk (0 to 3 risk factors) or high risk (4 to 5 risk factors) when assessing candidates for CNS prophylaxis.[6] It is important to note that these patients were treated in the pre-rituximab era. There is debate about whether use of rituximab leads to a reduction of CNS relapse risk, since systemic administration of rituximab has poor CNS penetration. It is generally believed that improved systemic disease control with rituximab leads to improved outcomes, but it may not prevent CNS relapse in cases of occult CNS involvement at time of diagnosis.[3,4]
The National Comprehensive Cancer Network (NCCN) guidelines recommend use of the CNS-International Prognostic Index (IPI). This prognostic model is based on data from 2,164 patients in the German High-Grade NHL Study Group (DSHNHL) originally published in 2013.[7,8] It allows one to stratify patients into low- (0 to 1 factors, estimated CNS disease rate < 1%), intermediate- (2 to 3 factors, estimated CNS disease rate 2% to 10%), and high-risk (4 to 6 factors, estimated CNS disease rate > 10%) categories for CNS relapse. The model is based on the following 6 characteristics, the first 5 of which make up the IPI scoring system: age > 60 years, elevated serum LDH level, performance status > 1, stage III or IV disease, extranodal sites > 1, and kidney or adrenal gland involvement. Updated results published in 2016 isolated patients with DLBCL from the DSHNHL dataset and demonstrated 2-year rates of CNS relapse to be 10% in the high-risk group compared with 0.8% and 2.9% in the low- and intermediate-risk groups, respectively.[8] This prognostic model was validated when applied to a British Columbia Cancer Agency dataset of patients with DLBCL (Table 1).[8,9] This study recommends a CNS diagnostic workup including lumbar puncture with cytology and flow cytometry for intermediate- and high-risk patients and consideration of CNS-directed prophylactic therapies for high-risk patients.[9]
A recent prognostic model developed by Kanemasa et al retrospectively stratified 413 patients into low- (0 to 2 factors) or high-risk (3 to 4 factors) groups based on 4 characteristics: extranodal sites > 1, low serum albumin level, retroperitoneal lymph node involvement, and stage III or IV disease. CNS relapse occurred in 27 patients (6.5%), and 62 (15%) received IT prophylaxis.[10] When applied to high-risk patients, this model captured higher 5-year CNS relapse rates than the Hollender and DSHNHL models (26.4% vs 18.3% and 17.6%, respectively). Additionally, 5-year CNS relapse rates for low-risk patients were lower than those of the Hollender and DSHNHL models (3.0% vs 5.6% and 3.5%, respectively). However, the model developed by Kanemasa et al has not been validated, was evaluated in a much smaller patient population than other models, and involved a single institution.[10]
Table 1 summarizes the key features of existing prognostic models for CNS relapse and the datasets used to derive these models. While all models discussed previously are practical for use in clinical practice, the model proposed by Schmitz et al and recommended by NCCN guidelines has the strongest evidence for use as it is the most validated.[8] Furthermore, wider use of this model may facilitate comparisons between studies.
Despite the ability of these prognostic models to increase the specificity of predicting CNS relapse, relying solely on their use to determine who should receive CNS prophylaxis could exclude a large number of patients who will ultimately experience relapse. At the same time, approximately 80% of patients classified as high risk who are administered prophylaxis will not relapse, resulting in the overtreatment of some. Therefore, it is essential to consider additional risk factors to use in combination with prognostic models to avoid over- or undertreatment.
Additional risk factors
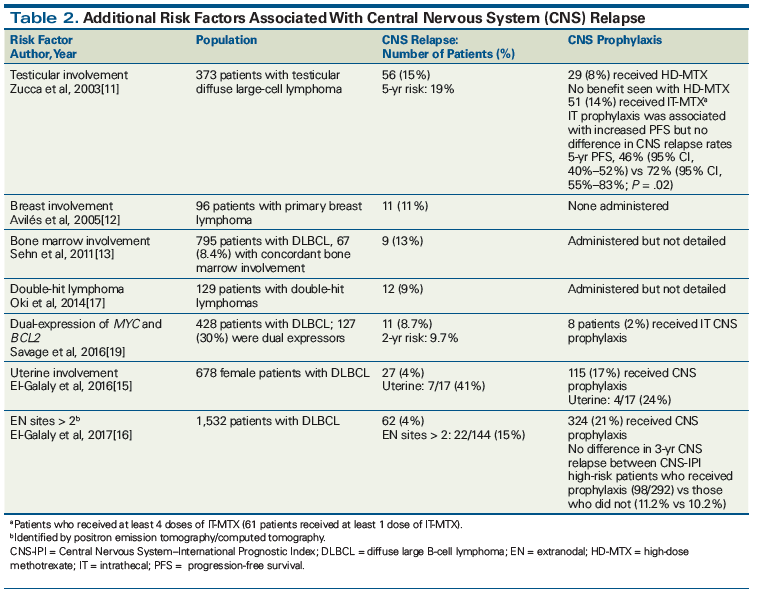
When assessing individual patient risk for CNS relapse, additional clinical risk factors that the prognostic models do not specifically address must be considered. These risk factors, as derived from retrospective studies, include a diagnosis of primary lymphoma of the breast, bone marrow involvement, testicular involvement, uterine (but not ovarian) involvement, and involvement of more than two extranodal sites (Table 2).[11-16] While sinonasal involvement has historically been associated with an increased risk of CNS relapse, more recent studies refute early findings and report CNS relapse rates at 2% to 4%.[14]
Biomarkers have also been associated with CNS relapse risk. DLBCLs with dual translocations of MYC and BCL2 or BCL6, termed double-hit lymphomas, are associated with high rates of CNS involvement. An MD Anderson Cancer Center review of 129 patients with double-hit lymphomas found that the incidence of CNS involvement was 13% at 3 years, and only 4% of patients were diagnosed with CNS disease at presentation.[17] Another review of 311 patients with double-hit lymphomas found that 7% of patients were diagnosed with CNS disease at baseline, although it did not report the number of patients who experienced CNS relapse.[18] Furthermore, dual expressors of MYC and BCL2 or BCL6 by immunohistochemistry have demonstrated an increased risk of relapse vs non–dual expressors.[19] A recent review of 428 patients showed that the 2-year CNS relapse risk was 9.7% in dual expressors vs 2.2% in non–dual expressors (P = .001). Significantly more patients with DLBCL are dual expressors (30%) than have double-hit lymphoma (5%), suggesting that more patients may be at risk for CNS relapse than previously thought. When coupled with the CNS-IPI, dual expressors in CNS-IPI intermediate- and high-risk groups were found to have a 2-year risk of CNS relapse of 11% and 22.7%, respectively.[19]
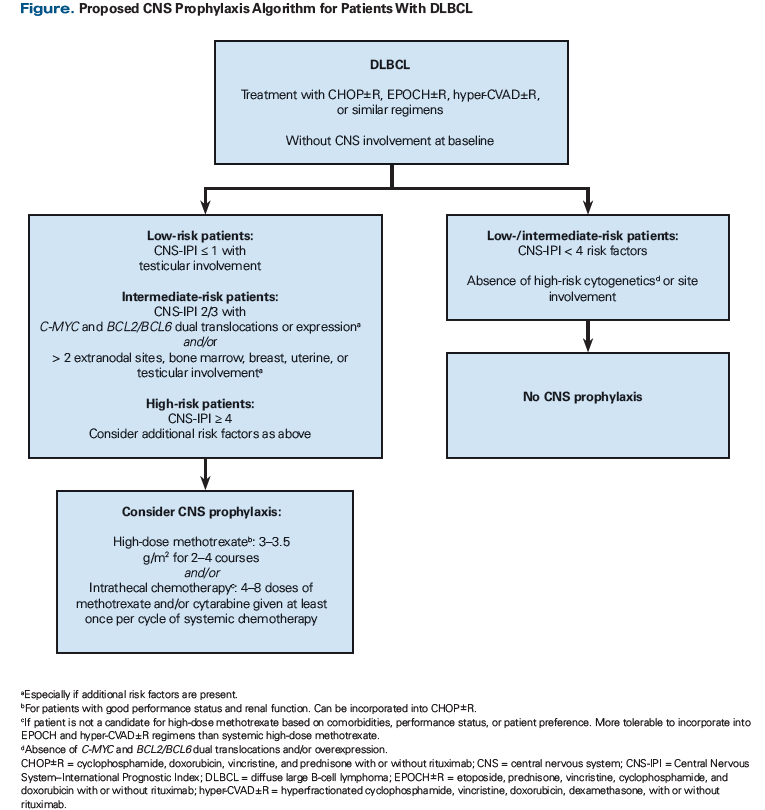
Table 2 also describes additional clinical factors associated with increased risk of CNS relapse and the datasets used to identify them. In light of these data, the current methods used to predict CNS relapse need refinement before an ideal population of high-risk patients can be identified. The goal is twofold: to identify patients who will benefit sufficiently from CNS prophylaxis to warrant the additional toxicity of therapy, and to identify patients in need of prophylaxis based on other clinical considerations. Although it remains to be formally studied, using validated prognostic models together with individual clinical and biological risk factors may enable clinicians to achieve this goal and identify patients at greatest risk for CNS involvement. The Figure displays a strategy for combining the CNS-IPI with additional clinical and biological risk factors to identify DLBCL patients at increased risk for CNS relapse and in need of CNS prophylaxis.
CNS Prophylaxis: Which Route Is Best?
Intrathecal prophylaxis
If CNS prophylaxis is to be administered, the NCCN guidelines recommend IT methotrexate and/or cytarabine or systemic (ie, intravenous) methotrexate.[7] IT methotrexate and/or cytarabine is the most common prophylaxis and is often incorporated into the most frequently used DLBCL chemotherapy regimens-ie, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab). While many studies have either defended or refuted the benefit of CNS prophylaxis, they were limited due to their retrospective nature, small patient populations, or lack of control groups.[20,21] Further, it is difficult to discern the effect that CNS prophylaxis had in many of these studies, as the highest-risk patients were more often administered prophylaxis, thus confounding the results due to selection bias. Therefore, we recommend following the NCCN guidelines.[7]
Arguably, the RICOVER-60 study offers some of the most intriguing evidence to date regarding CNS prophylaxis, due to a large number of protocol violations.[4] While CNS prophylaxis with 4 doses of IT methotrexate was required for patients with lymphoma involvement of the bone marrow, testes, or upper head and neck, 90 patients (43%) did not receive prophylaxis despite possessing one of the specified risk factors. Despite this, there was a nonsignificant difference in the rate of relapse between the group given prophylaxis and those who did not receive it (2.5% vs 4.4%).[4] However, considering the risk factors included in the prognostic models previously reviewed, it is likely that many of these patients may not have been as high risk as once thought.
The impact of IT prophylaxis in combination with R-CHOP chemotherapy and radiation was assessed by the International Extranodal Lymphoma Study Group in a prospective phase II trial of patients with stage I or II primary testicular lymphoma.[22] Fifty of 53 patients (94%) received planned CNS prophylaxis with 4 doses of IT methotrexate during the first 2 cycles of R-CHOP. Three of the 10 relapses occurred in the CNS, resulting in a 5-year CNS relapse incidence of 6%. While it is difficult to discern the effect that IT prophylaxis may have provided in the context of concurrent treatment modalities, this study had a lower incidence of CNS relapse compared with previous studies.[11,22] Additionally, this study demonstrated the relative tolerability of IT prophylaxis, as only 3 patients discontinued planned prophylaxis due to intolerance or toxicities.[22]
When IT prophylaxis is used, the NCCN guidelines recommend administering 4 to 8 doses of methotrexate and/or cytarabine.[7] While the most effective number of doses and drug combinations have not been thoroughly studied, it seems appropriate to administer prophylaxis as early as possible in the disease course to derive the greatest benefit, since most relapses occur soon after diagnosis.[2-4] Although no firm recommendation can be made, IT prophylaxis beginning with a diagnostic lumbar puncture followed by administration with each cycle of systemic chemotherapy or as often as twice weekly for at least 4 doses has been common practice.[4,23]
IT prophylaxis is unlikely to prevent parenchymal relapse, as pharmacokinetic studies of intrathecally administered methotrexate and cytarabine demonstrate poor distribution into the brain parenchyma.[24] This is unfortunate, since most CNS relapses in the rituximab era involve the brain parenchyma.[25] Still, IT prophylaxis does have a potential role in high-risk patients; an example would be patients whose performance status indicates that they could not tolerate a more intensive chemotherapy regimen that could adequately penetrate the blood-brain barrier and distribute into the brain parenchyma. Though generally well-tolerated, patients receiving IT prophylaxis may experience significant side effects, such as chemical arachnoiditis, headache, and cerebrospinal fluid (CSF) leak.[20,23,24] Thus, the potential benefits of IT CNS prophylaxis must be weighed against these risks.
Systemic prophylaxis
As previously stated, there is concern that IT methotrexate and/or cytarabine is not adequate prophylaxis for prevention of CNS relapse.[7] A comparison of methotrexate distribution based on route of administration-including intravenous, lumbar puncture, and ventricular via Ommaya reservoir-demonstrated highly variable methotrexate concentrations in the CSF after administration via lumbar puncture. In contrast, methotrexate concentrations were greater and more evenly distributed after intravenous and intraventricular administration.[26] In addition, there are data demonstrating the effectiveness of systemic high-dose methotrexate.[27] CSF levels of methotrexate correlate with the serum concentration of free methotrexate, and higher levels of non-protein-bound drug can be achieved with rapid infusions.[28,29] Therefore, it is important to consider the dose and infusion duration of high-dose methotrexate, as lower doses of 1 g/m2 or prolonged infusions of 8 g/m2 over 24 hours do not routinely reach cytotoxic levels in the CSF.[29-31] Clinical studies have demonstrated cytotoxic CSF levels of methotrexate with doses > 3 g/m2 administered over 2 to 3 hours.[30,31] Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) is often used for aggressive disease, including Burkitt lymphoma and lymphoblastic lymphoma. Occasionally administered in high-risk patients with DLBCL, hyper-CVAD alternates with high-dose methotrexate and high-dose cytarabine.[32-34] However, the total systemic methotrexate dose of 1 g/m2 is likely not adequate by itself for CNS prophylaxis, and patients receiving hyper-CVAD still require IT chemotherapy if CNS prophylaxis is desired.[34]
KEY POINTS
- Current prognostic models aid clinicians in the selection of patients with diffuse large B-cell lymphoma who are at highest risk for central nervous system (CNS) relapse, although the ideal selection of these patients still remains a challenge.
- Additional individual clinical and biological risk factors, used along with validated prognostic models, may enhance the selection of patients at greatest risk for CNS involvement.
- Systemic and intrathecal methods of chemotherapy prophylaxis should be considered for high-risk patients based on patient-specific risk factors and comorbidities.
Several retrospective and prospective studies provide evidence for the use of systemic CNS prophylaxis. Abramson et al retrospectively evaluated the addition of high-dose methotrexate as systemic CNS prophylaxis in high-risk DLBCL on day 15 of R-CHOP at a dose of 3.5 g/m2.[27] The 65 patients included in the analysis received a median of 3 cycles of high-dose methotrexate. Only 2 patients experienced CNS relapse, which was lower than expected for this patient group.[27] Twenty-six patients experienced renal toxicity at some point during their treatment course. Chemotherapy was delayed in 8 patients due to methotrexate toxicity, including renal dysfunction, mucositis, and cytopenias. There was one death associated with chemotherapy toxicity.[27] Cheah et al conducted a multicenter, retrospective analysis of 217 DLBCL patients at high risk for CNS relapse. High risk was defined as two or more of the following: multiple extranodal sites; elevated serum LDH level; and B symptoms or specific sites of involvement, such as bone marrow, breast, testis, kidney, or adrenal glands.[35] There were 3 treatment groups: IT methotrexate with R-CHOP (group 1), R-CHOP with IT methotrexate and 2 cycles of high-dose methotrexate (group 2), and hyper-CVAD or CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate; alternating with ifosfamide, etoposide, and cytarabine) with IT methotrexate (group 3). Three-year CNS relapse rates were similar between groups 2 and 3 (6.9% vs 2.3%; P = .16) but significantly higher in group 1 (18.4%; P = .009). Per a univariate analysis, only treatment group was related to CNS relapse risk. While these results provide evidence that supports systemic prophylaxis, it is important to note that over 80% of patients in groups 2 and 3 also received at least 1 dose of IT prophylaxis.[35] Ferreri et al retrospectively evaluated 200 patients in a risk-tailored approach to CNS prophylaxis in patients who had DLBCL.[36] Patients received CNS prophylaxis with systemic high-dose methotrexate with or without IT chemotherapy. Patients were categorized by CNS-IPI as either low (47%) or high risk (53%). Forty high-risk patients diagnosed after 2007 were eligible for CNS prophylaxis; 33 received high-dose methotrexate for 3 or 4 cycles with or without IT chemotherapy and 7 patients received IT chemotherapy alone. Only one patient who received adequate CNS prophylaxis experienced CNS relapse (this patient could not tolerate high-dose methotrexate because of renal dysfunction and received IT chemotherapy alone). In contrast, 8 patients (12%) who did not receive CNS prophylaxis experienced CNS relapse. There were no reported CNS relapses in patients who received high-dose methotrexate as CNS prophylaxis.[36]
The Groupe d’Ãtudes des Lymphomes de l’Adulte (GELA) prospectively compared CHOP (n = 312) vs ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone; n = 323) with IT methotrexate in patients with aggressive NHL in the pre-rituximab era.[37] Patients in the ACVBP arm received consolidation chemotherapy with 2 cycles of high-dose intravenous methotrexate (3 g/m2), 4 cycles of etoposide and ifosfamide with mesna, and 2 cycles of subcutaneous cytarabine. No CNS prophylaxis was administered to patients in the CHOP arm. There were significantly more cases of CNS relapse in the CHOP arm than in the ACVBP arm that included CNS prophylaxis (26 vs 9 patients). Patients receiving the more intensive chemotherapy experienced higher rates of toxicity, including myelosuppression and infection, and there were 43 treatment-related deaths in the ACVBP arm compared with 23 in the CHOP arm. However, the GELA authors concluded that the reduced CNS relapse rate and better overall survival justified the use of more intensive chemotherapy and CNS prophylaxis.[37]
Holte et al assessed systemic CNS prophylaxis in 156 patients aged 18 to 65 years with higher-risk DLBCL.[38] After completing 6 cycles of rituximab, cyclophosphamide, doxorubicin, etoposide, and prednisone every 14 days (R-CHOEP-14), systemic CNS prophylaxis included high-dose cytarabine, 3 g/m2 twice daily for 2 days (2 g/m2 for patients aged 60 to 65), followed 3 weeks later by high-dose methotrexate, 3 g/m2 infused over 24 hours (1.5 g/m2 for patients aged 60 to 65). There were 3 deaths related to chemotherapy toxicity. Seven patients (4.5%) experienced CNS relapse, all of which occurred within the first 6 months of study inclusion. Holte et al concluded that, while the rate of CNS relapse was lower than expected, earlier incorporation of prophylaxis may yield even greater benefit in preventing early CNS relapse.[38]
Which DLBCL patients should be considered for systemic high-dose methotrexate with or without IT chemotherapy for CNS prophylaxis? As shown in the Figure, patients with dual-expressor status and those with a high-risk CNS-IPI score, an intermediate-risk CNS-IPI score with high-risk cytogenetics and/or specific site involvement, or a low-risk CNS-IPI score with testicular involvement treated with R-CHOP (or a similar regimen) are candidates. Although the ideal dose and schedule have not been established, methotrexate, 3 to 4 g/m2 for 2 to 4 courses, should be considered. It is also unclear whether IT chemotherapy should be included in CNS prophylaxis, given that many studies included a combination of modalities. Patients with DLBCL and double-hit lymphoma should also be considered for CNS prophylaxis with systemic high-dose methotrexate. However, the optimal means for incorporating systemic high-dose methotrexate into intensive front-line chemoimmunotherapy remains to be elucidated. Tolerance and toxicities associated with high-dose methotrexate must be considered prior to initiating prophylaxis. Systemic therapy may not be possible in patients with renal dysfunction or poor performance status. High-dose methotrexate administration also requires hospital admission for sodium bicarbonate fluid infusion for urine alkalization, leucovorin administration, and methotrexate level monitoring.[27,35]
Conclusion
Clearly, there is an unmet need for novel approaches to risk stratification, prophylaxis methods, and diagnostic tools related to CNS relapse in patients with DLBCL. Considering the most recent evidence, systemic prophylaxis with high-dose methotrexate is likely the most effective prophylaxis but is associated with additional toxicities. The prophylaxis strategy offered by Abramson et al[27] may be the best fit for high-risk patients with good performance status receiving R-CHOP. However, an optimal systemic prophylaxis that fits well into more intense chemotherapy regimens such as EPOCH and hyper-CVAD remains to be discovered. The Figure displays a practical algorithm that incorporates additional clinical risk factors, as well as effective strategies in fit patients for addressing CNS relapses that are most likely to occur in the brain parenchyma. Such approaches may assist clinicians in determining which patients are at highest risk for CNS relapse and thus may benefit from prophylaxis.[21,23,38,39]
It is also important to consider physician experience, institutional practices, and patient preference, which cannot be captured by this algorithm. Because randomized trials incorporating CNS prophylaxis are unlikely to be performed due to ethical and logistical concerns, additional retrospective data in large patient cohorts are necessary to clarify these unresolved questions. The validation and enhancement of previously described prognostic models and prophylaxis methods with additional evidence will be paramount in refining current guideline recommendations.
Financial Disclosure:Dr. Flowers is a consultant for AbbVie, Bayer, Celgene (unpaid), Denovo Biopharma, Genentech/Roche (unpaid), Gilead, Karyopharm, OptumRx, Pharmacyclics/Janssen, and Spectrum; he has received research funding from AbbVie, Acerta, Burroughs Wellcome Fund, Celgene, Eastern Cooperative Oncology Group, Genentech/Roche, Gilead, Janssen Pharmaceutical, Millennium/Takeda, National Cancer Institute, Pharmacyclics, TG Therapeutics, and V Foundation. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016 Sep 12. [Epub ahead of print]
2. Bernstein SH, Unger JM, Leblanc MJ, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 - the Southwest Oncology Group. J Clin Oncol. 2009;27:114-9.
3. Tai WM, Chung J, Tang PL, et al. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol. 2011;90:809-18.
4. Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113:3896-902.
5. Zahid MF, Khan N, Hashmi SK, et al. Central nervous system prophylaxis in diffuse large B-cell lymphoma. Eur J Haematol. 2016;97:108-20.
6. Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin's lymphoma: a risk model. Ann Oncol. 2002;13:1099-107.
7. National Comprehensive Cancer Network. Non-Hodgkin’s lymphoma. Version 5.2017. http://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed October 1, 2017.
8. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34:3150-6.
9. Savage K, Zeynalova S, Kansara R, et al. Validation of a prognostic model to assess the risk of CNS disease in patients with aggressive B-cell lymphoma. Blood. 2014;124:abstr 394.
10. Kanemasa Y, Shimoyama T, Sasaki Y, et al. Central nervous system relapse in patients with diffuse large B cell lymphoma: analysis of the risk factors and proposal of a new prognostic model. Ann Hematol. 2016;95:1661-9.
11. Zucca E, Conconi A, Mughal T, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21:20-7.
12. Avilés A, Delgado S, Nambo MJ, et al. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005;69:256-60.
13. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29:1452-7.
14. Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood Rev. 2006;20:319-32.
15. El-Galaly TC, Cheah CY, Hutchings M, et al. Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol. 2016;175:876-83.
16. El-Galaly T, Villa D, Michaelsen TY, et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: an international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer. 2017;75:195-203.
17. Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891-901.
18. Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354-61.
19. Savage K, Kerry J, Slack G, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127:2182-8.
20. McMillan A, Ardeshna KM, Cwynarski K, et al. Guideline on the prevention of secondary central nervous system lymphoma: British Committee for Standards in Haematology. Br J Haematol. 2013;163:168-81.
21. Schmitz N, Nickelsen M, Savage KJ. Central nervous system prophylaxis for aggressive B-cell lymphoma: who, what, and when? Hematol Oncol Clin North Am. 2016;30:1277-91.
22. Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29:2766-72.
23. Ghose A, Kundu R, Latif T. Prophylactic CNS directed therapy in systemic diffuse large B cell lymphoma. Crit Rev Oncol Hematol. 2014;91:292-303.
24. Fleischhack G, Jaehde U, Bode U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin Pharmacokinet. 2005;44:1-31.
25. Kansara R, Villa D, Gerrie AS, et al. Site of central nervous system (CNS) relapse in patients with diffuse large B-cell lymphoma (DLBCL) by the CNS-IPI risk model. Br J Haematol. 2017;179:508-10.
26. Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293:161-6.
27. Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116:4283-90.
28. Evans WE, Hutson PR, Stewart CF, et al. Methotrexate cerebrospinal fluid and serum concentrations after intermediate-dose methotrexate infusion. Clin Pharmacol Ther. 1983;33:301-7.
29. Khan RB, Shi W, Thaler HT, et al. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58:175-8.
30. Hiraga S, Arita N, Ohnishi T, et al. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg. 1999;91:221-30.
31. Vassal G, Valteau D, Bonnay M, et al. Cerebrospinal fluid and plasma methotrexate levels following high-dose regimen given as a 3-hour intravenous infusion in children with nonHodgkin’s lymphoma. Pediatr Hematol Oncol. 1990;7:71-7.
32. Muringampurath-John D, Jaye DL, Flowers CR, et al. Characteristics and outcomes of diffuse large B-cell lymphoma presenting in leukaemic phase. Br J Haematol. 2012;158:608-14.
33. Chihara D, Cheah CY, Westin JR, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15 year follow up of a phase II study from MD Anderson Cancer Center. Br J Haematol. 2016;172:80-8.
34. Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569-80.
35. Cheah CY, Herbert KE, O’Rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer. 2014;111:1072-9.
36. Ferreri AJ, Bruno-Ventre M, Donadoni G, et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol. 2015;168:654-62.
37. Tilly H, Lepage E, Coiffier B, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102:4284-9.
38. Holte H, Leppä S, Björkholm M, et al. Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol. 2013;24:1385-92.
39. Savage KJ. Secondary CNS relapse in diffuse large B-cell lymphoma: defining high-risk patients and optimization of prophylaxis strategies. Hematology Am Soc Hematol Educ Program. 2017;2017:578-86.