Rituximab/Cyclophosphamide/Dexamethasone Combination Highly Effective in Autoimmune Hemolytic Anemia Associated With Chronic Lymphocytic Leukemia
The exact mechanism of development of autoimmune hemolytic anemia (AIHA) in chronic lymphocytic leukemia (CLL) is unclear, but the imbalance among lymphocyte subsets is considered to be the basis for the emergence of an autoimmune
The exact mechanism of development of autoimmune hemolyticanemia (AIHA) in chronic lymphocytic leukemia (CLL) is unclear, but theimbalance among lymphocyte subsets is considered to be the basis for theemergence of an autoimmune clone. Treatment of this complication is aimed atimmunosuppression with glucocorticosteroids as first-line treatment. Oncesteroid therapy fails there is no known effective treatment, although variousagents are commonly used with variable response rates. We present our resultswith a combination of drugs targeting the B lymphocytes, in addition to otherlymphocyte populations.
Eight CLL patients with Coombs-positive AIHA, who had failedsteroid therapy, were treated between December 1998 and March 2000. Thetreatment regimen consisted of rituximab (Rituxan) given at a dose of 375 mg/m2IV infusion on day 1, cyclophosphamide (Cytoxan, Neosar) at a dose of 750 to1,000 mg/m2 IV on day 2, and dexamethasone at 12 mg/d IV on days 1 and 2 andorally from days 3 to 7. This treatment was repeated monthly for 4 months, or itwas stopped sooner if a maximally achievable beneficial response was reached.The table below provides the pre- and posttreatment absolute lymphocyte count(ALC) × 1,000/µL, hemoglobin (Hb) in g/dL, and platelets × 1,000/µL:
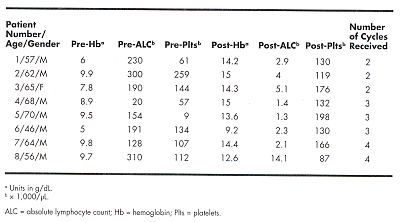
In five patients the Coombs test became negative aftertreatment. All patients had a dramatic response, with all but one patientachieving normal Hb levels. The median duration of response in AIHA is in excessof 12 months, and only one patient had a relapse (at 11 months). In addition toAIHA, all patients showed a marked improvement in their underlying CLL. Apartfrom the first-infusion-related transient reactions with rituximab, there wereno undue toxicities.
CONCLUSION: These results demonstrate that our rituximab-basedregimen is extremely effective, not only for controlling AIHA, but also theunderlying CLL.
Click here to read Dr. Bruce Cheson's commentary on this abstract.