The understanding of the relationship between genetic variation and an individual patient’s response to radiation therapy (RT) has gained significant ground over the past several years. Genetic markers have been identified that could ultimately serve as the foundation for predictive models in clinical practice, and that hold the potential to revolutionize the delivery of precision medicine in oncology. Single nucleotide polymorphisms, single genes, and/or gene signatures could ultimately serve as the basis for patient stratification in prospective clinical trials. Currently, molecular markers relevant to breast, lung, and head and neck cancers have been integrated into clinical practice and serve as predictive tools to guide systemic therapy. In the future, the use of predictive models based on genomic determinants may become standard practice in radiation oncology, offering the potential to further personalize the delivery of RT and optimize the therapeutic ratio.
Introduction
Radiation therapy (RT) remains a mainstay of modern oncologic treatment, with more than half of all patients receiving RT during their treatment course. However, individual responses to RT vary widely among disease types and patient populations.[1] Recent years have been marked by the development and expanded use of precision medicine in cancer therapeutics. Precision medicine refers to the tailoring of treatment to the individual characteristics of each patient, based on inherent susceptibilities. Although enormous strides have been made in tailoring a variety of approaches to systemic therapy, the role of radiation oncology in precision medicine is just beginning to emerge.[1]
Precision in RT has been advancing along multiple parallel paths. There have been improvements in the precision of anatomic target delineation with the use of intensity-modulated RT, volumetric arc therapy, and stereotactic RT, all of which allow for improved target dose conformality. Concurrent with technical advances in treatment delivery, the field of radiogenomics, or the interplay between genomic elements and radiation response at the cellular level, continues to evolve. Indexing the determinants of radiation response at the cellular level has the potential to allow for more personalized delivery of RT and to further increase the therapeutic ratio of our treatment.[1]
As the rates of cancer survival continue to improve, the effect of treatment toxicity on normal tissue will play an increasingly important role in treatment selection. Capturing patient-reported outcomes from the growing and evolving survivor population sheds light on the potential far-reaching impact of radiogenomics beyond traditional survival measures. Specifically, by recognizing the connection between genotypic variation and normal tissue response, our ability to predict severe toxicities following RT may spare selected individuals from significant morbidity and mortality following treatment.[2] Moreover, studies investigating genetic assays predictive of tumor radiosensitivity may be complementary to studies evaluating the radiosensitivity of noncancerous tissue.[3] The purpose of the current article is multifold: Herein, we will review the background and history of genomic predictors of RT response; evaluate candidate genes and polymorphisms dictating responses to radiation; discuss emerging data on the use of genetic signatures; and review current guidelines on the use of genomic predictors to tailor therapy. The article is structured to discuss outcomes and toxicities based on precision medicine in RT within each of these sections.
Background and History
Prognostic vs predictive markers in oncology
Biomarkers have long been used in the field of oncology as an adjunct to traditional staging information to estimate treatment outcomes. In this field of study, it is important to distinguish between prognostic and predictive biomarkers. Prognostic markers are associated with a clinical outcome, such as overall survival (OS), regardless of the treatment delivered.[4] For example, the prostate-specific antigen (PSA) has been proven to be an important biomarker in prostate cancer, correlating with the risk of recurrence and OS. Although elevated PSA levels are associated with worse outcomes, measurement of PSA alone does not yet predict the patient response to specific treatments.
Predictive markers, on the other hand, are indicators of the likely benefit following specific treatment. These markers are therefore useful in tailoring treatment decisions. An example of a predictive marker is ERBB2 gene amplification (resulting in overexpression of human epidermal growth factor receptor 2 [HER2]) in breast cancer, since clinical outcomes are improved by the addition of trastuzumab to the chemotherapy regimen in patients with this genetic aberration.[4] The National Comprehensive Cancer Network (NCCN), in updating the NCCN Biomarkers Compendium, recently released a task force report addressing the use of molecular biomarkers in six major disease sites.[5] While prognostic biomarkers provide important information regarding clinical outcome, implicit to the goal of precision medicine is the identification of predictive biomarkers to help direct individual treatment. Despite the significant progress made by radiogenomics in this regard over the past 20 years-from focused gene studies to genome-wide association studies (GWAS)-in the field of radiation oncology, clinical translation of these principles remains a goal on the horizon.[2]
Initial discovery of discrepant radiation responses
Studies investigating variable responses of tissues to RT date back more than 60 years ago to the investigations carried out by Gray and colleagues.[6-8] Specifically studied was the effect of oxygenation on RT response. The tumor microenvironment has been demonstrated to have topographic variability; certain regions possess particularly low extracellular pH, low nutrient content, and hypoxia. Given the often tortuous and malformed vasculature of tumors, blood flow to the microenvironment contributes to an imbalance in the supply of and demand for oxygen. The resulting hypoxia correlates with tumor cell radioresistance, since the maximal effect of RT is achieved by the generation of free radicals.[6] Preceding the early discovery of the effect of hypoxia on radioresistance was the demonstration of individual variation in the response of normal tissue following treatment with a given dose of radiation. This was first formally described in 1936 with the publication of the now well-described sigmoid dose–response curve.[9] Alongside the discovery of differing individual responses to similar radiation doses was the detection of RT hypersensitivity in patients with certain rare genetic syndromes. The first such documented adverse reaction occurred in a 10-year-old patient with mutation of the ATM gene, who died from complications related to radiation toxicity in normal tissues. Since this initial case was reported, the ATM mutation has been intricately linked with the DNA damage response and studied extensively.[10] While the demonstration of radiosensitivity in patients with rare genetic disorders has been instrumental in our understanding of differential radiation responses, it does not yet explain the wide range of radiation responses seen in patients without known genetic syndromes.
Genetic Profiling in the RT Clinical Domain
Background and history
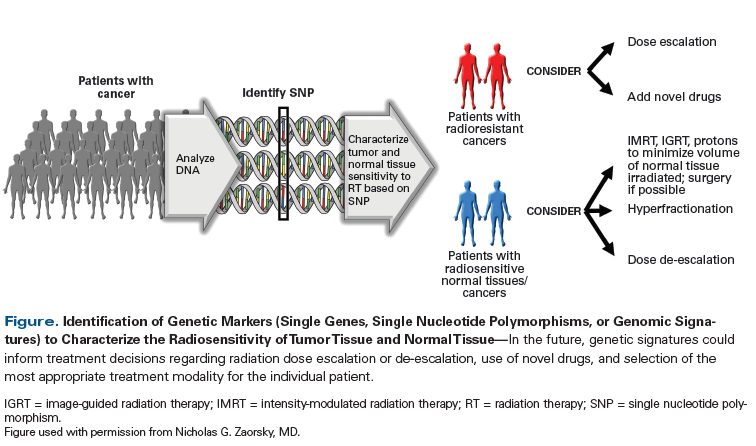
Identification of genetic markers (either single genes, single nucleotide polymorphisms [SNPs], or genomic signatures) for response to RT is underway and could ultimately form the foundation for predictive models in the radiation oncology clinic (Figure). Investigation of the relationship between radiosensitivity and tumor-specific genes such as KRAS and EGFR, as well as gene signatures (such as those recently identified in breast cancer and prostate cancer), hold promise for revolutionizing precision medicine in radiation oncology.
Radiation response in the NCI-60 and development of the radiosensitivity index (RSI)
In the 1980s, the National Cancer Institute (NCI) investigated 60 cell lines representing 9 tumor types for in vitro analysis of drug mechanisms and cancer biology; these are known as the NCI-60 Human Tumor Cell Lines Screen. More than 100,000 chemotherapy agents have been tested in these cell lines.[11] In the mid 2000s, Torres-Roca et al sought to identify genes correlated with RT response in 35 of the NCI-60 cell lines; they assessed the genetic contributions to radiosensitivity by quantifying the surviving fraction following exposure of the cell lines to a standard radiation dose of 2 Gy (the SF2 assay). The authors expressed concerns about reproducibility of their initial studies due to the lack of in vivo validation.[12] However, Eschrich et al subsequently expanded the model to 48 of the NCI-60 cell lines and included biological variables such as the mutation status of RAS and TP53, as well as tissue of origin.[13] Using these data, a rank-based linear algorithm was created to calculate a radiosensitivity index (RSI). The RSI has now been evaluated in independent cohorts spanning multiple tissue lineages, and its predictive potential continues to be tested.[13-15]
The RSI has a demonstrated ability to predict for 5-year progression-free survival (PFS) and 5-year distant metastasis–free survival in two distinct NCI breast cancer database sets.[14] The survival benefit was noted in patients treated with RT who were found to be radiosensitive (defined as an RSI above the 25th percentile) compared with those who were found to be radioresistant (RSI below the 25th percentile). No difference in survival was noted between radiosensitive vs radioresistant patients who were not treated with RT, further supporting the predictive value of the RSI as it relates to radiation treatment.
Ahmed et al have similarly demonstrated the predictive values of the RSI in glioblastoma; in a population of 270 patients, they found it to be an independent predictor of OS.[16] Further studies, ideally prospective ones, would be important to fully validate the index for routine clinical use.
Gene studies and genome-wide association studies
A unifying early theme among researchers was the detection of SNPs, which are associated with development of radiation-induced toxicity in normal tissue.[17] This research led to significant associations reported for SNPs in a variety of candidate genes, but in large part these associations could not be validated by subsequent studies.[17-19] In response to the often-contradictory initial study results reported, experts in the fields of radiation oncology, radiobiology, medical oncology, and epidemiology joined together to form the Radiogenomics Consortium (established in Manchester, United Kingdom, in 2009), with two initial goals: (1) to make an assay predictive of radiation toxicity in patients undergoing standard RT; and (2) to define molecular pathways responsible for normal tissue toxicity through identification of genes with SNPs associated with these adverse effects.[19] Due to the inherent limitations of candidate gene studies, under the direction of this consortium the focus has shifted to GWAS in an effort to survey multiple genes (even those not previously associated with RT response).[2]
Targeting Specific Pathogens, Genes, and Pathways
Human papillomavirus (HPV) in head and neck cancer
HPV-related head and neck cancer is an emerging clinical entity, with specific HPV variants now associated with up to 50% to 75% of cancers arising from the oropharynx.[20] The disease course of HPV-positive cancer of the oropharynx is different from that of patients with classic smoking-related head and neck cancer.[21] The superior response to RT observed in patients with HPV-related cancers has been hypothesized to be due to the increased radiosensitivity of HPV-related cancers.[22,23] Excellent outcomes reported in these patients have spurred a growing interest in de-escalating the intensity of treatment. Unfortunately, there are only a few established HPV-positive cell lines available for investigation, limiting the ability of current research to establish the true radiosensitive nature of this disease entity.[24]
Given the significant morbidity and toxicity associated with therapy for head and neck cancer, there is growing enthusiasm to investigate several approaches to treatment de-escalation; two large multi-institutional trials are now evaluating de-escalation of the radiation dose and/or chemotherapy agents used (ClinicalTrials.gov identifiers: NCT01898494, NCT02254278). In North America alone, in addition to the aforementioned studies, multple studies with de-escalation protocols are investigating this matter.[25]
In the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network 3311 trial (ClinicalTrials.gov identifier: NCT01898494), patients will be stratified by risk groups following minimally invasive surgery. Patients with intermediate-risk disease will be randomized to a radiation dose of 50 Gy or 60 Gy postoperatively. In the NRG Oncology Group HN002 trial (ClinicalTrials.gov identifier: NCT02254278), investigators will use p16 status as a surrogate for HPV-related oropharynx cancer and will randomize patients to intensity-modulated RT 5 days a week for 6 weeks to a total dose of 60 Gy with weekly cisplatin vs RT to 60 Gy alone. The results of these ongoing trials are not yet available; therefore, incorporation of HPV status into treatment decisions, while holding great promise, has not been established.
KRAS in head and neck cancer
In many cancers, activating mutations of KRAS have been established as a biomarker indicating poor prognosis. While KRAS mutations are rare in head and neck cancer (< 5%), a germline, functional SNP in the 3'-untranslated region of KRAS has been shown to alter the activity of wild-type KRAS. This variant has been shown to have prognostic and predictive significance in recurrent head and neck cancer treated with chemotherapy. Specifically, KRAS-activating mutations have been associated with resistance to cisplatin.[26] Recent studies have evaluated the relationship between KRAS mutations and radioresistance. The presence of single allelic mutations was demonstrated to induce radioresistance in vivo, and KRAS G13D mutations were associated with radioresistance in a mouse model. Moreover, KRAS-mutant cells demonstrate the following functions, all associated with radioresistance: increased DNA damage repair at the nuclear level, reduced mitotic catastrophe following RT, and early escape from post-RT G2/M cell-cycle arrest.[27]
EGFR in head and neck cancer and lung cancer
Epidermal growth factor receptor (EGFR) and HER2 both belong to the ERBB receptor family (and are designated by ERBB1 and ERBB2, respectively). Receptor amplification and gain-of-function mutations are the most common alterations of EGFR and occur in several types of malignancies, including lung cancer and head and neck cancer.[28,29] Amplification of the ERBB2 gene is the most common type of aberration and typically results in overexpression of HER2, a condition most commonly encountered in breast cancer.[30] The identification of genetic changes, such as EGFR mutations, in certain malignancies has led to the development of targeted therapies for the relevant patient populations. Specifically, cetuximab is approved by the US Food and Drug Administration (FDA) for the treatment of head and neck squamous cell carcinoma (HNSCC), which is associated with mutation of EGFR.
Ongoing studies aim to optimize clinical outcomes with combination therapy that incorporates targeted therapy, including trials of targeted therapy, such as cetuximab, plus RT. In a phase III clinical trial conducted by Bonner et al, cetuximab administered concurrently with RT yielded a survival benefit in patients with HNSCC; in addition, locoregional control in patients treated with the cetuximab-plus-RT combination was 24.4 months, compared with 14.9 months (hazard ratio [HR], 0.68; P = .005) in patients treated with RT alone.[31] In current practice, patients with locally advanced head and neck cancer typically receive cetuximab if they are medically unfit for chemotherapy; however, there have been no head-to-head trials comparing outcomes with cetuximab combined with chemoradiotherapy vs cetuximab combined with RT. In their study, Bonner et al concluded that the benefit of cetuximab in head and neck cancer is likely attributable to the radiosensitization effects of this EGFR-targeting agent.[31] To date, however, no biomarker has been identified to predict which patients will respond best to cetuximab alone or which will have better radiosensitivity to treatment with cetuximab plus RT.
While EGFR alteration in head and neck cancer tends to correlate with worse outcomes, EGFR alteration in patients with lung cancer portends an improved prognosis.[1] Patients with EGFR-mutated lung cancers have shown high sensitivity to the EGFR kinase inhibitors gefitinib and erlotinib since their introduction into daily clinical practice beginning with their FDA approvals in 2003 and 2004, respectively.[32] Based on multiple randomized controlled trials (RCTs), the NCCN Guidelines for Non–Small-Cell Lung Cancer now call for EGFR testing as a category 1 recommendation.[33] Ongoing trials are continuing to explore differences in the relationship between EGFR alterations and the response to treatment, as well as the correlative vs causative nature of radiosensitivity in patients with EGFR mutations.[1]
Molecular classification of gliomas
Previous classification of gliomas was based on pathologic features alone, including necrosis, atypia, and mitotic figures. The World Health Organization classification of central nervous system tumors released in 2016 now incorporates molecular parameters for the first time.[34] Eckel-Passow et al published pivotal work analyzing and attempting to restratify gliomas (grades II to IV) based on analysis of TERT, IDH, and SNPs of multiple additional genes.[35] This retrospective analysis provided prognostic information (with reported 5-year OS rates of 5% in patients whose tumors were TERT-mutated only vs 70% in those with only IDH–mutated tumors vs 90% in patients with mutations of both TERT and IDH). An additional retrospective study by The Cancer Genome Atlas Research Network reported genomic analysis of gliomas (grades II to IV), in addition to more comprehensive stratification of survival curves based on molecular signatures, and showed that patients with IDH wild-type low-grade gliomas have a prognosis similar to that of patients with IDH-mutant glioblastoma, with both groups having an estimated 5-year OS rate of 20%.[36]
Several large RCTs have grouped patients into study arms based on molecular markers, such as the Radiation Therapy Oncology Group (RTOG) 9802 study, which stratified patients with low-grade glioma by IDH mutation status, and the European Organisation for Research and Treatment of Cancer (EORTC) 22033-26033 trials, which stratified patients by 1p/19q codeletion status.[37,38] Prospectively, these trials have demonstrated the prognostic significance of such markers, setting the stage for further clinical trial development and customization of therapy based on tumor genomics to potentially demonstrate predictive patterns. In a subgroup analysis of EORTC 22033-26033, Baumert et al evaluated the relationship between various molecular markers and the response to treatment with temozolomide plus RT in patients with high-risk low-grade glioma. Among all comers, there was no difference in PFS between groups. In a post-hoc subgroup analysis, the authors evaluated the impact of IDH mutation status, 1p/19q status, and MGMT methylation status on patient outcomes. The presence of IDH mutation (regardless of 1p/19q status) was found to be a positive prognostic factor; patients with IDH-mutated tumors who were 1p/19q intact had an improved PFS in response to the RT-plus-temozolomide regimen, compared with those who received temozolomide alone (HR, 1.86; 95% CI, 1.21–2.87; P = .0043).[38]
Although subset analyses of prospective trials do give insight into molecular correlations and potential predictive significance, using this genomic knowledge to advance precision medicine in the clinic still requires significant exploration. Phase I and II trials are underway (ClinicalTrials.gov identifiers: NCT02968940, NCT02977689) that investigate treatment based on the patient’s IDH mutation status; however, the interplay between genomics and molecular therapies in combination with RT may not be as straightforward as researchers had initially envisioned.
Gene Signatures
Radiosensitivity signature in breast cancer
RT continues to be a mainstay of treatment for both early-stage breast cancer, following breast conservation surgery, and locally advanced breast cancer, following mastectomy. Given the significant reduction in rates of locoregional recurrence and breast cancer mortality at 15 years in these populations, as shown in a meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group, adjuvant RT remains the standard of care for most women.[39] Molecular subtyping in breast cancer has made tremendous strides over the past several years, and has the potential to guide treatment interventions. Barriers to widespread use of complete genetic analysis have been the cost of tissue processing and the expertise required. As a result, immunohistochemistry staining of molecular surrogates (eg, the estrogen receptor, progesterone receptor, and HER2) has been employed to help clinicians define molecular subtypes.[40]
The use of personalized adjuvant treatment in breast cancer has been pioneered using the 21-gene recurrence score assay (Oncotype DX). This decision-making tool discerns the risk of distant disease in patients with estrogen receptor–positive, lymph node–negative breast cancer treated with tamoxifen, and its use has been expanded to predict the benefit of chemotherapy in these patients.[1,41]
KEY POINTS
- Concurrent with technical advances in radiation treatment delivery, the field of radiogenomics, or the interplay between genomic elements and response to radiation at the cellular level, continues to evolve.
- Despite the significant progression of radiogenomics over the past 20 years-from focused gene studies to genome-wide association studies-in the field of radiation oncology, clinical translation of these principles remains a goal on the horizon.
There is growing interest in the use of similar predictive tools to determine the benefit of adjuvant RT. Recent work by Speers et al reveals the development and validation of a novel radiosensitivity signature (RSS) in patients with breast cancer achieved by integrating post-RT clonogenic survival data with gene expression data across breast cancer cell lines.[42] Using these assays, they identified a range of radiosensitivities in breast cancer cells that did not significantly correlate with the intrinsic cancer subtype. Specifically, 147 genes were found to correspond with radiosensitivity. The RSS was further refined to 51 genes specifically involving cell-cycle arrest and DNA repair mechanisms. After independent dataset validation, on multivariate analysis, the signature was found to be the most significant factor in predicting local recurrence. This genetic signature outperformed all clinicopathologic features currently considered in patient management, and may potentially be used to identify patients who would benefit from RT intensification vs those at low risk of recurrence following standard RT.[42] Further development of this tool may make it possible to select for patients who will most likely benefit from postoperative RT; the ability to make this distinction is important given the potentially significant impact of RT on quality of life, cosmesis, and disease-specific outcomes.
Radiosensitivity signature in cervical cancer
Cervical cancer is the second most common cause of cancer death among women in developing countries. In the United States, despite the availability of screening with the Papanicolaou-or “Pap”-smear, more than 12,000 new cases of invasive cervical cancer were estimated for 2016, with more than 4,000 cervical cancer–related deaths.[43] The standard of care for locally advanced cervical cancer includes concurrent chemoradiotherapy, but currently molecular information is not used to guide individualization of treatment.[44-46] Tissue samples from the RTOG 0128 study, a phase II trial evaluating the benefit of celecoxib with cisplatin chemotherapy and RT for locally advanced cervical cancer, were analyzed to evaluate the potential of gene expression signatures as predictors of treatment response. Gene expression profiling was performed prior to initiation of treatment and midway through the treatment regimen (following chemoradiotherapy but before brachytherapy with radioactive implants inserted into the tumor site), with evaluation of alterations in gene expression patterns between these two time points. Of the 22 patients with available tissue for analysis, a changed gene expression signature (consisting of 7 genes) between pretreatment and midtreatment biopsies predicted treatment response. This 7-gene signature separated patients with local failures from those who achieved local control.[47] Other genome-wide expression analyses have been reported in cervical cancer; however, there has not yet been one predictive set of genes identified and validated.[48-50] Therefore, the use of genetic signatures to inform the treatment of cervical cancer is still in its infancy; nevertheless, methodology standardization may ultimately lead to the validation and clinical utilization of these decision-making tools.[51]
Radiosensitivity signature in prostate cancer
RT is commonly used for the treatment of localized prostate cancer, and three RCTs have demonstrated the benefit of adjuvant RT given post prostatectomy in enabling patients to achieve biochemical control.[52-54] In the Southwest Oncology Group 8794 trial, adjuvant RT was found to significantly improve metastasis-free survival, the primary endpoint of this study, at 15 years.[55] Multiple other retrospective studies evaluating salvage RT have also suggested that it reduces biochemical recurrence (ranging from 24% to 66% at 5 years).[56-58] As with other disease sites, directing treatment through predictive gene signatures would be particularly useful in the setting of biochemical recurrence following prostatectomy. A recently published study by Zhao et al introduced a 24-gene signature that was predictive of response to postoperative RT in prostate cancer. This signature was developed in a matched training prostatectomy cohort of 196 patients. The 24-gene Post-Operative Radiation Therapy Outcomes Score (PORTOS) was developed using ridge-penalized Cox regression as a way to model interactions between genes and RT. A similarly matched cohort of 330 patients was used for the validation study. Treated patients with a higher PORTOS had lower rates of metastases than their untreated counterparts (5% vs 63% at 10 years; P < .0001). In contrast, patients with a lower PORTOS who were untreated had lower rates of metastases than their treated counterparts (31% vs 57%, respectively, at 10 years; P = .0001).[59]
Ultimately, expanded development of robust and reproducible gene signatures for use in the management of prostate cancer will continue to improve the personalization of treatment. The PORTOS genomic signature is an example of one novel tool in this area with a clinical-grade platform. As Zhao et al noted in their study, future directions include developing and validating specific signatures for different post-prostatectomy scenarios, since the PORTOS signature did not distinguish between adjuvant and salvage RT.[59]
Guidelines in Radiogenomics
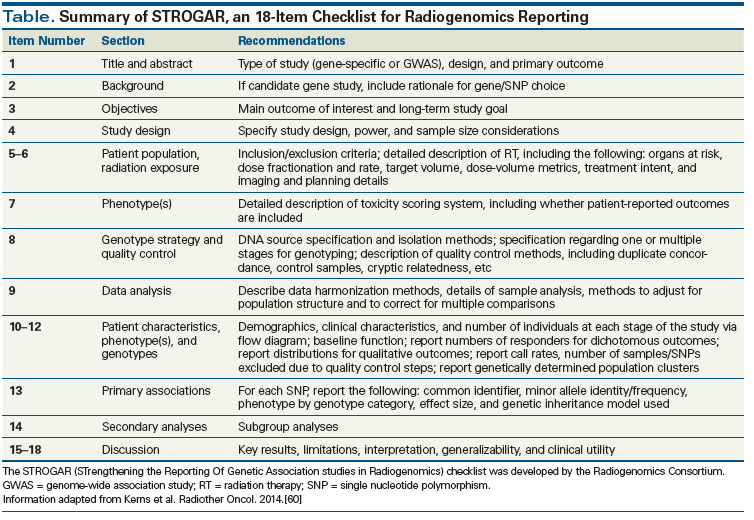
The Radiogenomics Consortium recently released guidelines encouraging transparency in the reporting of data, aimed at improving the overall quality of radiogenomics research. STROGAR (STrengthening the Reporting Of Genetic Association studies in Radiogenomics) is comprised of an 18-item checklist for radiogenomic study reporting, which is summarized in the Table.[60] Prior to the development of STROGAR, the CONSORT (Consolidated Standards of Reporting Trials) Statement[61] aimed to clarify the quality of reporting, including determinants of internal and external validity. The STROBE Statement (STrengthening the Reporting of OBservational Studies in Epidemiology) subsequently provided recommendations for the accurate and complete reporting of biomedical observational studies.[62] STREGA (STrengthening the REporting of Genetic Association Studies; an extension of the STROBE Statement)[63] provides additional guidelines specific to genetic association studies.
Conclusion
The study of the impact of genetic variation on RT response, more commonly known as “radiogenomics,” has gained significant ground over the past several years. Radiogenomics continues to hold promise for use in daily clinical practice, and it is anticipated that further advances in this field will have a meaningful impact on patient outcomes and quality of life. Identifying genetic markers that are able to ultimately predict an individual patient’s response to RT will allow for further customization and precision of treatment at an individual level. SNP-based models could ultimately serve as the basis for stratification in RT trials. Molecular markers relevant for treatment decision making in breast, lung, and head and neck cancers have been integrated into clinical practice by serving as surrogates for underlying genetic differences leading to variable responses to RT in both tumor and healthy tissue.
It is the goal of the Radiogenomics Consortium to establish reliable and predictive models based on GWAS to facilitate the adjustment of treatment paradigms according to genetic predispositions found to confer either radiosensitivity or radioresistance.
Financial Disclosure:Dr. Den is the recipient of a research grant from GenomeDx. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Yard B, Chie EK, Adams DJ, et al. Radiotherapy in the era of precision medicine. Semin Radiat Oncol. 2015;25:227-36.
2. Kerns SL, West CM, Andreassen CN, et al. Radiogenomics: the search for genetic predictors of radiotherapy response. Future Oncol. 2014;10:2391-406.
3. Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134-42.
4. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216.
5. Febbo PG, Ladanyi M, Aldape KD, et al. NCCN Task Force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9(suppl 5):S1-S32.
6. Hill RP, Bristow RG, Fyles A, et al. Hypoxia and predicting radiation response. Semin Radiat Oncol. 2015;25:260-72.
7. Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638-48.
8. Gray LH. Radiobiologic basis of oxygen as a modifying factor in radiation therapy. Am J Roentgenol Radium Ther Nucl Med. 1961;85:803-15.
9. Holthusen H. Erfahrungen über die Verträglichkeitsgrenze für Röntgenstrahlen and deren Nutzanwendung zur Verhütung von Schäden. Strahlentherapie. 1936;57:254-69.
10. Gotoff SP, Amirmokri E, Liebner EJ. Ataxia telangiectasia: neoplasia, untoward response to x-irradiation, and tuberous sclerosis. Am J Dis Child. 1967;114:617-25.
11. Amundson SA, Do KT, Vinikoor LC, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68:415-24.
12. Torres-Roca JF, Eschrich S, Zhao H, et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res. 2005;65:7169-76.
13. Eschrich S, Zhang H, Zhao H, et al. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys. 2009;75:497-505.
14. Eschrich SA, Fulp WJ, Pawitan Y, et al. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18:5134-43.
15. Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75:489-96.
16. Ahmed KA, Chinnaiyan P, Fulp WJ, et al. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget. 2015;6:34414-22.
17. Barnett GC, Coles CE, Elliott RM, et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol. 2012;13:65-77.
18. Sachidanandam R, Weissman D, Schmidt SC, et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928-33.
19. West C, Rosenstein BS, Alsner J, et al. Establishment of a radiogenomics consortium. Int J Radiat Oncol Biol Phys. 2010;76:1295-6.
20. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612-9.
21. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35.
22. Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791-800.
23. Rieckmann T, Tribius S, Grob TJ, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242-6.
24. Kimple RJ, Harari PM, Torres AD, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumor grafts. Clin Cancer Res. 2013;19:855-64.
25. Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50:2636-48.
26. Chung CH, Lee JW, Slebos RJ, et al. A 3’-UTR KRAS-variant is associated with cisplatin resistance in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2014;25:2230-6. Erratum in: Ann Oncol. 2015;26:1038-9.
27. Williams TM, Yang L, Estrada A, et al. KRAS oncogenic mutations induce intrinsic resistance to radiation through up-regulation of DNA repair pathways. Int J Radiat Oncol Biol Phys. 2016;96:S238.
28. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543-50.
29. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576-82.
30. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61-70.
31. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21-8. Erratum in: Lancet Oncol. 2010;11:14.
32. Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005;23:2556-68.
33. National Comprehensive Cancer Network. Non-small cell lung cancer. Version 4.2016. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed September 4, 2016.
34. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803-20.
35. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372:2499-508.
36. Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481-98.
37. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30:3065-70.
38. Baumert BG, Hegi ME, Mason WP, et al. Radiotherapy in relation to temozolomide: subgroup analysis of molecular markers of the randomized phase III study by the EORTC/NCIC-CTG/TROG/MRC-CTU (EORTC 22033-26033) in patients with a high risk low-grade glioma. J Clin Oncol. 2015;33(suppl):abstr 2006.
39. Darby S, McGale P, Correa C, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16.
40. Huber KE, Carey LA, Wazer DE. Breast cancer molecular subtypes in patients with locally advanced disease: impact on prognosis, patterns of recurrence, and response to therapy. Semin Radiat Oncol. 2009;19:204-10.
41. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817-26.
42. Speers C, Zhao S, Liu M, et al. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin Cancer Res. 2015;21:3667-77.
43. American Cancer Society. Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016.
44. Morris M, Eifel P, Lu J. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137-43.
45. Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966-72.
46. Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based chemoradiation improves progression free and overall survival in advanced cervical cancer: results of a randomized Gynecologic Oncology Group study. N Engl J Med. 1999;340:1144-53.
47. Weidhaas JB, Li SX, Winter K, Ryu J, et al. Changes in gene expression predicting local control in cervical cancer: results from Radiation Therapy Oncology Group 0128. Clin Cancer Res. 2009;15:4199-206.
48. Choi YW, Kim YW, Bae SM, et al. Identification of differentially expressed genes using annealing control primer-based GeneFishing in human squamous cell cervical carcinoma. Clin Oncol (R Coll Radiol). 2007;19:308-18.
49. Grigsby PW, Watson M, Powell MA, et al. Gene expression patterns in advanced human cervical cancer. Int J Gynecol Cancer. 2006;16:562-7.
50. Klopp AH, Jhingran A, Ramdas L, et al. Gene expression changes in cervical squamous cell carcinoma after initiation of chemoradiation and correlation with clinical outcome. Int J Radiat Oncol Biol Phys. 2008;71:226-36.
51. Klopp AH, Eifel PJ. Biological predictors of cervical cancer response to radiation therapy. Semin Radiat Oncol. 2012;22:143-50.
52. Wiegel T, Bartkowiak D, Bottke D, et al. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse–free survival and overall survival: 10-year data of the ARAO 96-02 trial. Int J Radiat Oncol Biol Phys. 2015;91:288-94.
53. Bolla M, van Poppel H, Collette L, et al; European Organisation for Research and Treatment of Cancer. Postoperative radiotherapy after radical prostatectomy: a randomized controlled trial (EORTC trial 22911). Lancet. 2005;366:572-8.
54. Thompson IM Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329-35.
55. Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956-62.
56. Neuhof D, Hentschel T, Bischof M, et al. Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:1411-7.
57. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163:845-50.
58. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325-32.
59. Zhao SG, Chang SL, Spratt DE, et al. A 24-gene predictor of response to postoperative radiation therapy in prostate cancer. Int J Radiat Oncol Biol Phys. 2016;96(suppl):S104-S105.
60. Kerns SL, de Ruysscher D, Andreassen CN, et al. STROGAR-STrengthening the Reporting Of Genetic Association studies in Radiogenomics. Radiother Oncol. 2014;110:182-8.
61. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996;276:637-9.
62. von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-7. Erratum in: Ann Intern Med. 2008;148:168.
63. Little J, Higgins JP, Ioannidis JP, et al. STrengthening the REporting of Genetic Association studies (STREGA): an extension of the STROBE Statement. Ann Intern Med. 2009;150:206-15.