Correct Answers:
1: False
2: False
A 70-year-old man presented at our institution for a second opinion regarding diagnosis of a urinary bladder mass. He had a 3-year history of worsening urinary incontinence and urgency, for which he had undergone colonoscopy, as well as testing for prostate issues; all test results were negative.
Oncology (Williston Park). 31(3):210-212, 218-220.

Figure 1. CT Scans of the Urinary Bladder at Time of First Diagnosis and Before Transurethral Resection
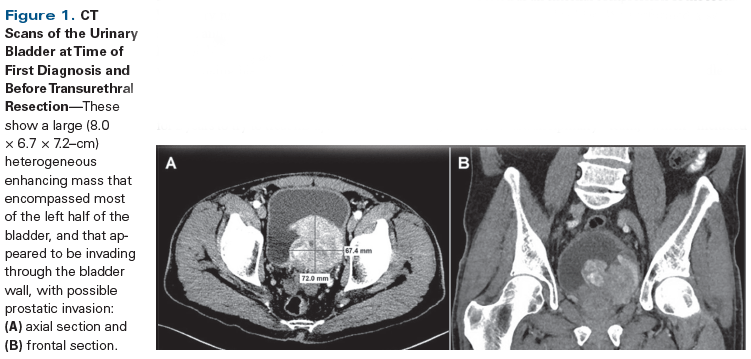
Figure 2. Gross Pathology of the Urinary Bladder Tumor
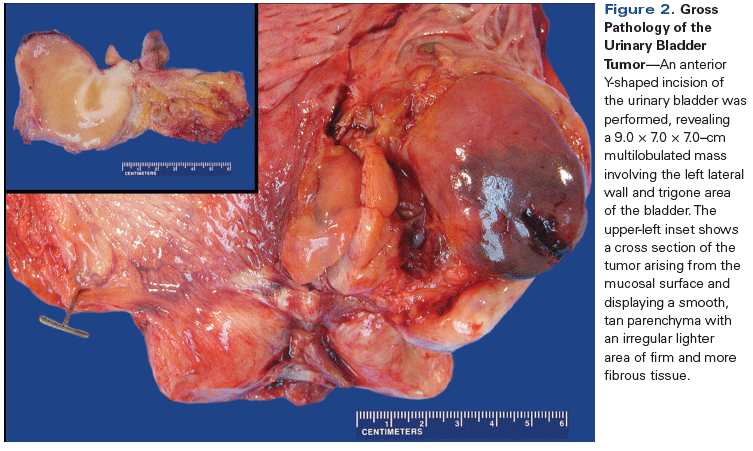
Figure 3. Histologic Images of the Spindle Cell Neoplasm
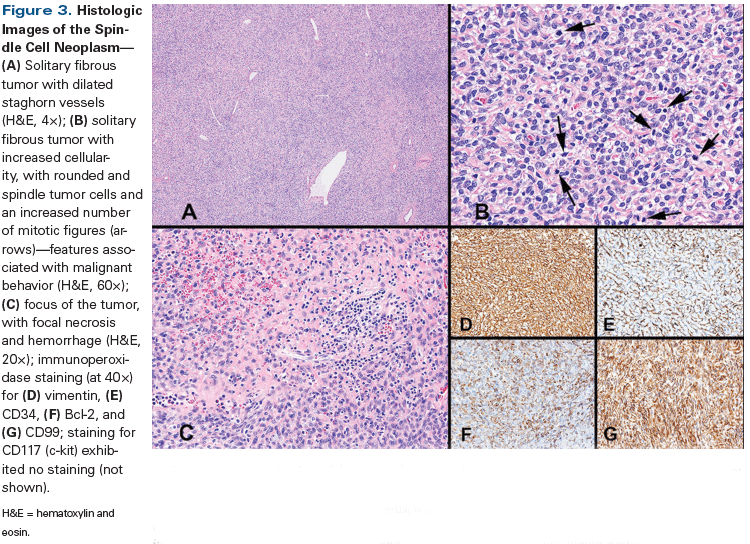
Table 1. Summary of Literature Review Results
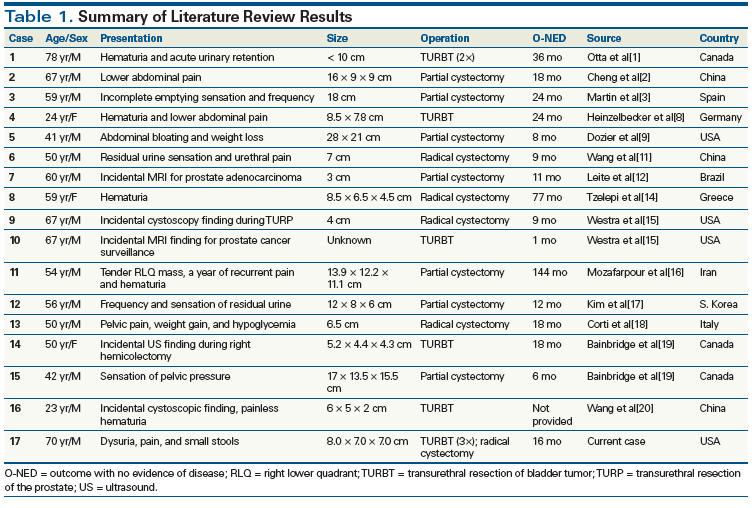
Table 2. Risk Stratification Model
A 70-year-old man presented at our institution for a second opinion regarding diagnosis of a urinary bladder mass. He had a 3-year history of worsening urinary incontinence and urgency, for which he had undergone colonoscopy, as well as testing for prostate issues; all test results were negative. He experienced some improvement with tamsulosin. One month prior to admission, he was seen at an outside clinic for left flank pain; dysuria; gross hematuria; and intermittent episodes of diarrhea and constipation, with small stools. An outside chest/abdominal/pelvic CT scan with and without contrast showed a large, 8.0 × 6.7 × 7.2–cm, heterogeneous enhancing mass that encompassed most of the left half of the bladder and appeared to be invading through the bladder wall, with possible prostatic invasion (Figure 1). There was bilateral, mild ureterectasis, but no definitive hydronephrosis. No evidence of regional adenopathy or other suspicious distant lesion was observed. Significant laboratory findings included an elevated prostate-specific antigen (PSA) level of 5.6 ng/mL and a low hemoglobin level of 8.2 g/dL. He had a 20-pack-year smoking history and a family history significant for bladder cancer in his father. He had been taking a homeopathic colloidal silver medication for 2 years to try to treat his dysuria, and as a result, his partial thromboplastin time (PTT) was significantly elevated (42 to 60 seconds).
The differential diagnosis included a primary urothelial carcinoma, as well as other malignancies, such as lymphoma, sarcoma, and metastatic cancer-and benign pathologies, such as blood clots and bladder stone(s). A cystoscopic examination with partial transurethral resection of bladder tumor (TURBT), performed at the referring facility, confirmed the presence of a bladder mass, and pathologic examination of the TURBT specimen yielded a diagnosis of solitary fibrous tumor. After receiving this diagnosis, the patient started using strong neodymium magnets that he would place on his abdomen, which he believed helped alleviate some of his symptoms and helped prevent cancer progression.
When the patient was admitted to our facility, digital rectal examination revealed an extrinsic palpable mass pressuring the left side; this was interpreted as an external compression of the rectum by an enlarged bladder. We performed a second opinion pathology examination of the original specimen from the outside facility; the diagnosis, based on this pathology exam, was a spindle cell tumor with associated scar tissue, consistent with solitary fibrous tumor.
A multidisciplinary team, which included urologists, medical oncologists, radiation oncologists, and pathologists, recommended radical cystoprostatectomy, since the tumor was not amenable to partial cystectomy because of its location. However, the patient refused this radical approach and he chose to attempt additional homeopathic colloidal silver therapy. After using this alternative therapy for more than a month, with worsening of the urinary symptoms, he sought additional consultation with the medical oncology service. Neoadjuvant chemotherapy was not recommended due to a lack of standard indications for chemotherapy[1-3]; instead, complete resection of the tumor was recommended as having the best prognosis.
Two months after the first TURBT, we performed a second cystoscopy, which showed the bladder almost entirely filled by a very large tumor (approximately 9 cm in maximal dimension) arising from the left bladder wall. Because the patient refused a radical resection of the tumor, we performed a debulking procedure in which the tumor was resected layer by layer. A significant amount of the tumor was not resected because of its size and because of the patient’s known coagulopathy. The resected tumor fragments were sent to our pathology laboratory.
Histopathology examination of this second TURBT revealed a spindle cell neoplasm with histologic features and immunophenotype very similar to those of the previous specimen, but with the addition of atypical features consistent with a malignant nature. These findings were confirmed after consultation with two other in-house pathologists, and the consensus diagnosis was a malignant solitary fibrous tumor, invasive into fragments of muscularis propria, consistent with pathology stage pT2.
Based on these results, the patient finally accepted the recommendation to proceed with radical cystoprostatectomy with bilateral pelvic lymph node dissection and creation of a continent Vescica Ileale Padovana pouch. At the time of surgery, the tumor was found to be significantly larger than it had been at the time of his last cystoscopy, extending throughout the bladder from the prostate to the urachus. It measured 9 cm across the pelvis, with extension into the prostatic urethra.
Gross examination of the bladder revealed a 9.0 × 7.0 × 7.0–cm multilobulated mass involving most of the left lateral wall and trigone area of the bladder (Figure 2). Cross section of the tumor showed a smooth, tan parenchyma with an irregular lighter area of firm and more fibrous tissue.
Microscopic examination of the tumor showed histopathologic features that were very similar to those of the previous two TURBT specimens, with dilated staghorn blood vessels, increased cellularity of rounded and spindle tumor cells, and an increased number of mitotic figures (up to 8 per 10 high-power fields [HPF]). Small foci of necrotic tumor with hemorrhage were also present. The tumor had invaded deep into the muscularis propria-up to the outer half of the detrusor muscle-with a pathology stage of pT2b. Immunophenotyping of the tumor cells (Figure 3) demonstrated positive staining for vimentin, CD34, CD99, and Bcl-2, and negative staining for pan-cytokeratin (AE1/AE3), high-molecular-weight cytokeratin (CK5/6), CD117 (c-kit), p63, S100, and muscle-specific actin.
Examination of the prostate revealed a bilateral primary prostatic adenocarcinoma, Gleason grade 3+4 = 7 (grade group 2; grade 4, approximately 40%), involving approximately 20% of the tissue submitted, with extraprostatic extension (stage pT3a). The surgical margins were free of tumor, with a 0.5-cm distance between tumor and closest (deep) margin. Additional specimens obtained during the radical surgical procedure from the left distal ureter, right distal ureter, and urethral margin showed no evidence of malignancy. Histopathology from the lymph node dissections from the left obturator, left common iliac, left iliac, presacral, right obturator, right common iliac, and right external iliac lymph nodes showed a total of 21 lymph nodes with no evidence of malignant involvement.
1. Solitary fibrous tumors of the urinary bladder are rare and usually malignant.
2. Specific urinary tract symptoms make solitary fibrous tumors relatively easy to differentiate from other more common tumors of the urinary bladder.
Solitary fibrous tumors are rare neoplasms that include a variety of mesenchymal tumors; solitary fibrous tumors were first cited in the medical literature by Wagner in 1871.[4] Many years later (1931), Klemperer and Rabin described for the first time the clinical and pathologic features of solitary fibrous tumors; they originally identified them as “fibrous mesotheliomas” because they were commonly found in the lungs, respiratory tract, and mediastinum.[5] Over the years, these pleural tumors acquired several names, including benign mesothelioma, localized fibrous mesothelioma, submesothelial fibroma, pleural fibroma, and localized fibrous tumor. However, other less frequent locations (eg, salivary glands, orbit, liver, etc) were later identified and the term “solitary fibrous tumors” became preferred.
1: False
2: False
In 1942, Stout and Murray were the first to characterize a new rare and aggressive vascular tumor derived from mesenchymal cells,[6] with oval nuclei and scant cytoplasm, and featuring Zimmermann pericytes, which they identified as hemangiopericytomas.[7] These tumors had histopathologic features very similar to those of synovial sarcoma and solitary fibrous tumor. It was later-with the advent of immunohistochemistry-that a common fibroblastic origin was confirmed, and hemangiopericytomas are now thought to represent a cellular variant within the spectrum of solitary fibrous tumors.
Solitary fibrous tumors are usually well-circumscribed tumors with a gray-white cut surface. Microscopic examination reveals a neoplasm with a variety of growth configurations, classically described as a patternless or storiform arrangement of spindle cells with bland nuclear morphology intermixed with dense collagen.[8] The background stroma demonstrates variably prominent hyalinization, with a dilated, ramifying vascular network (so-called staghorn vessels). Immunostains are characteristically positive for vimentin, CD34, CD99, and Bcl-2. This tumor antigenic profile is very similar to that of gastrointestinal stromal tumors; additional staining for CD117 serves to differentiate between these two entities. At the molecular level, a recurrent NAB2-STAT6 gene fusion has recently been described in solitary fibrous tumors, and it is now recognized that aberrations in NAB2 and STAT6 are seen in most solitary fibrous tumors (both benign and malignant). Consequently, STAT6 immunostains are positive in solitary fibrous tumors.[9,10] Malignant solitary fibrous tumors are identified on the basis of their larger size (> 10 cm) and the presence of increased cellularity, cellular pleomorphism, and increased mitotic rate (> 4 per 10 HPF), as well as hemorrhage and/or necrosis.[2]
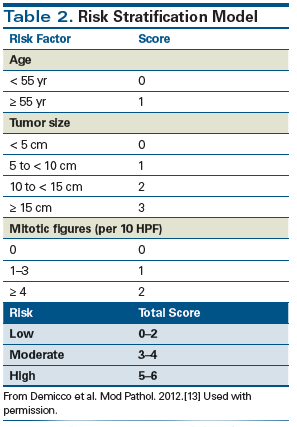
In extrapleural sites, solitary fibrous tumors are benign in 78% to 88% of cases and will metastasize or recur in only 10% to 20% of cases.[1,11] Urinary bladder occurrences of solitary fibrous tumors are extremely rare[12]; a comprehensive review of the literature reveals only 16 cases (Table 1), of which just 2 were malignant.[2,9] Thus, Statement 1 is false. A recent study by Demicco et al[13] proposed a risk stratification model for solitary fibrous tumors based on patient age, tumor size, and mitotic index to identify patients at high risk for poor clinical outcomes. They found that small tumors with low mitotic rates are highly unlikely to metastasize, while large tumors (≥ 15 cm) that occur in patients aged ≥ 55 years and that have mitotic figures ≥ 4 per 10 HPF require close follow-up and have a high risk of both metastasis and death.
Based on this risk stratification model (Table 2), our patient received 1 point for age (70 years old), 2 points for tumor size (9 cm), and 2 points for a mitotic rate of > 4 per 10 HPF. His total score of 5 points placed him in the high-risk category. The 5-year disease-specific survival rates in moderate- and high-risk groups were reported as 93% and 60%, respectively, while 10-year disease-specific survival rates were 93% and 0%, respectively.
Prompt treatment of a solitary fibrous tumor in the urinary bladder improves patient outcomes. The treatment currently indicated is a complete surgical excision of the tumor with careful follow-up.[14] There are no standard recommendations for chemotherapy or radiotherapy in the treatment of solitary fibrous tumors of the bladder, and the literature remains inconclusive concerning the efficacy of adjuvant therapy.[1-3] When a patient presents with a bladder tumor of mesenchymal origin, solitary fibrous tumor should be considered in the differential diagnosis and careful histopathologic examination must be performed to rule out the presence of malignant features.
We conducted a literature review using the search terms “spindle cell lesions of the bladder,” “malignant spindle cell lesions of the bladder,” “solitary fibrous tumor of the bladder,” and “malignant solitary fibrous bladder tumor.” The findings are listed in Table 1. Including our case study, there are 17 reported cases of solitary fibrous tumor of the urinary bladder; in 3 of these, the patients were female. The average age on presentation was 54 years.[1-3,8,9,11,12,14-20] Pain/pressure was the most commonly described symptom, which was reported in 7 of the patients (41.2%). Hematuria and urinary discomfort (dysuria, retention, or frequency) each were reported in 5 cases (29.4%). Notably, in 5 of the patients (29.4%) in our literature review, the solitary fibrous tumors were discovered incidentally via cystoscopy (2), MRI (2), or ultrasound (1). The general and nonspecific symptoms associated with solitary fibrous tumors of the urinary bladder make it difficult to differentiate these tumors from other more common bladder tumors. Thus, Statement 2 is false.
Seven patients (41.2%) in our literature review underwent a partial cystectomy, making this the most common treatment approach. Five patients (29.4%) chose radical cystectomy, and another five patients (29.4%) underwent at least one TURBT as their primary treatment. All these cases had positive survival outcomes, supporting the prompt, complete surgical removal of the solitary fibrous tumor as the most recommended treatment at this time.
The average length of reported follow-up was 26.9 months. For one patient, complete follow-up information was not available. The remaining 16 patients were without evidence of disease at last recorded follow-up, with 12 years being the longest length of disease-free follow-up.[16] These positive outcomes illustrate the importance of complete resection of solitary fibrous tumors of the bladder. TURBT would be a less invasive and less complicated approach than radical cystectomy, and could be considered as the first line of treatment, but mainly in cases where invasion of the detrusor muscle was not present. Although most solitary fibrous tumors of the bladder are benign,[17] upon discovery, immediate TURBT should be pursued to ensure positive outcomes and to minimize the chance of malignant transformation or metastasis (which ranges from 10% to 20%).[1]
Resectability is the most important prognostic factor in cases of solitary fibrous tumor of the bladder, followed by location, margins, size, and histopathologic appearance.[1] After resection, careful follow-up and surveillance are important.
A quick note regarding the use of colloidal silver: prolonged use of colloidal silver products has been found to cause coagulopathy, as evidenced in our case by the patient’s prolonged PTT (42 to 60 seconds).[21] The homeopathy industry recommends its use for different purposes, from killing various pathogens to promotion of tissue healing-and including prevention of urinary tract infections.[22] Also, a recent study suggests that colloidal silver has a dose-dependent cytotoxic effect on MCF-7 breast cancer cells.[23] However, even though the silver ion (Ag+) is bioactive in sufficient concentrations to kill bacteria in vitro, there is little evidence to support its medical use, and it is not recognized as safe or effective by the US Food and Drug Administration.[24]
Adjuvant chemotherapy and radiotherapy were not administered because of the tumor’s negative margins. The efficacy of adjuvant therapy in the management of solitary fibrous tumors remains controversial but is generally reserved for nonresectable, metastatic, and recurrent disease.[1-3] Six weeks after his cystoprostatectomy, the patient’s hemoglobin level had risen to 9.9 g/dL and his PSA level was < 0.01 ng/mL. After more than 1 year of follow-up, he is well and without recurrent, residual, or metastatic disease per MR and CT imaging performed at various times after the surgery.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
If you have a case that you feel has particular educational value, illustrating important points in diagnosis or treatment, you may send the concept to Dr. Crawford at david.crawford@ucdenver.edu for consideration for a future installment of Clinical Quandaries.
1. Otta RJ, Acosta MA, Gordillo C. A rare case of solitary fibrous tumour of the bladder. Can Urol Assoc J. 2014;8:E552–E553.
2. Cheng SH, Wang SS, Lee CH, et al. Malignant solitary fibrous tumor of the urinary bladder. J Chin Med Assoc. 2012;75:479-82.
3. Martin LL, Fernandez C. Solitary fibrous tumor of the bladder. Actas Urológicas Españolas. 2010;34:206-8.
4. Wagner EL. Das tuberkelähnliche Lymphadenom: (der cytogene oder reticulirte Tuberkel); mit zwei Tafeln. Wigand; 1871.
5. Klemperer P, Rabin CB. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22:4-31.
6. Stout AP, Murray MR. Localized pleural mesothelioma: investigation of its characteristics and histogenesis by method of tissue culture. Arch Pathol. 1942;34:951-64.
7. Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg. 1942;116:26-33.
8. Heinzelbecker J, Becker F, Pflugmann T, et al. Solitary fibrous tumour of the urinary bladder in a young woman presenting with haemodynamic-relevant gross haematuria. Eur Urol. 2008;54:1188-91.
9. Dozier J, Jameel Z, McCain DA, et al. Massive malignant solitary fibrous tumor arising from the bladder serosa: a case report. J Med Case Rep. 2015;9:46.
10. Tai H, Chuang I, Chen T, et al. NAB2–STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol. 2015;28:1324-35.
11. Wang T, Chen R, Qiao J, et al. Solitary fibrous tumor in bladder: a case report. J Huazhong Univ Sci Technolog Med Sci. 2010;30:412-4.
12. Leite K, Srougi M, Miotto A, Camara-Lopes LH. Solitary fibrous tumor in bladder wall. Int Braz J Urol. 2004;30:406-9.
13. Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298-306.
14. Tzelepi V, Zolota V, Batistatou A, Fokaefs E. Solitary fibrous tumor of the urinary bladder: report of a case with long-term follow-up and review of the literature. Eur Rev Med Pharmacol Sci. 2007;11:101-6.
15. Westra WH, Grenko RT, Epstein J. Solitary fibrous tumor of the lower urogenital tract: a report of five cases involving the seminal vesicles, urinary bladder, and prostate. Hum Pathol. 2000;31:63-8.
16. Mozafarpour S, Khorramirouz R, Tajali A, et al. Surgically treated bladder hemangiopericytoma/solitary fibrous tumor: report of a 12-year asymptomatic follow-up. Int Urol Nephrol. 2014;46:483-6.
17. Kim SH, Cha KB, Choi YD, et al. Solitary fibrous tumor of the urinary bladder. Yonsei Med J. 2004;45:573-6.
18. Corti B, Carella R, Gabusi E, et al. Solitary fibrous tumour of the urinary bladder with expression of bcl-2, CD32, and insulin-like growth factor type II. Eur Urol. 2001;39:484-8.
19. Bainbridge T, Singh R, Mentzel T, Katenkamp D. Solitary fibrous tumor of urinary bladder: report of two cases. Hum Pathol. 1997;28:1204-6.
20. Wang XM, Wang L. A case report of SFT in urinary bladder wall. J Diag Pathol. 2006;13:302-3.
21. Shrivastav S, Bera T, Singh SK, et al. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009;3:1357-64.
22. Maillard JY, Hartemann P. Silver as an antimicrobial: facts and gaps in knowledge. Crit Rev Microbiol. 2013;39:373-83.
23. Franco-Molina MA, Mendoza-Gamboa E, Sierra-Rivera CA, et al. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J Exp Clin Cancer Res. 2010;29:148.
24. Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev. 2010;(3): CD006478.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.