Strategies for Optimizing Molecular Testing in Non–Small Cell Lung Cancer
As genomic profiling becomes more and more essential as part of clinical decision making, clinicians are challenged to stay abreast of advancements in this space to improve patient outcomes and advance the field of precision oncology.

The advent of next-generation sequencing (NGS) has been a key driver in treatment advances and improved outcomes for patients with non–small cell lung cancer (NSCLC). However, implementation of universal molecular testing for patient populations who may benefit the most has been somewhat limited, with long turnaround times and lack of adequate tissue noted as 2 of the main causes.
Multiple clinical practice guidelines for physicians treating patients with this disease recommend that, at minimum, patients be tested for gene mutations in EGFR, ALK, and ROS1, as well as for expression of PD-L1. In addition, expanded panels that check for BRAF, MET, RET, HER2, and KRAS alterations are also advocated for in the literature given the actionability of these targets.1-3
Given the broad availability of approved therapeutic agents across multiple oncogenic targets, “hot spot” testing—testing that focuses on a specific gene alteration in NSCLC—is being supplanted by broader tumor profiling methods.
“Precision medicine works in NSCLC because of the large number of highly actionable oncogene targets,” said David R. Gandara, MD, director of thoracic oncology at the UC Davis Comprehensive Cancer Center in Sacramento, California, during a webinar for CancerNetwork®, the online home of the journal ONCOLOGY®. “If we consider the 8 actionable oncogenes…more than 50% of patients with nonsquamous NSCLC will have one of those, so the unknown has shrunk considerably.”
Moderator Edgardo S. Santos Castillero, MD, of Florida Precision Oncology in Aventura, and Mark G. Kris, MD, the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York, joined Gandara in discussing the implementation of early molecular testing for patients with NSCLC.
Available Agents for Tumors Harboring Mutations
In addition to immunotherapeutics that are approved to treat patients with high PD-L1 expression, such as single-agent pembrolizumab (Keytruda) for patients with metastatic tumors with expression by tumor proportion score of 1% or more, targeted therapies have transformed the systemic therapy landscape.
Most notably, tyrosine kinase inhibitors (TKIs) against EGFR are approved for use in both advanced disease and in adjuvant therapy for resectable tumors.
“Our job as thoracic oncologists is to identify this particular genomic alteration so we can provide to the patient the best therapy possible,” Santos Castillero said. “EGFR [as a therapeutic target] has been a great success. We have different TKIs and then also combinations—for example, erlotinib (Tarceva) plus ramucirumab (Cyramza).”
Santos Santos went on to detail ALK TKIs, for which there are currently 5 FDA-approved agents for use across patients in different settings for metastatic disease. Emerging more recently are targeted therapies against NTRK fusions, such as larotrectinib (Vitrakvi), and MET exon 14 skipping mutations, such as tepotinib (Tepmetko). These alterations are each present in about 3% of the overall NSCLC population.2 When they are identified, however, there's the potential for impressive therapeutic responses in patients who may have no other promising options.
Common Testing Platforms
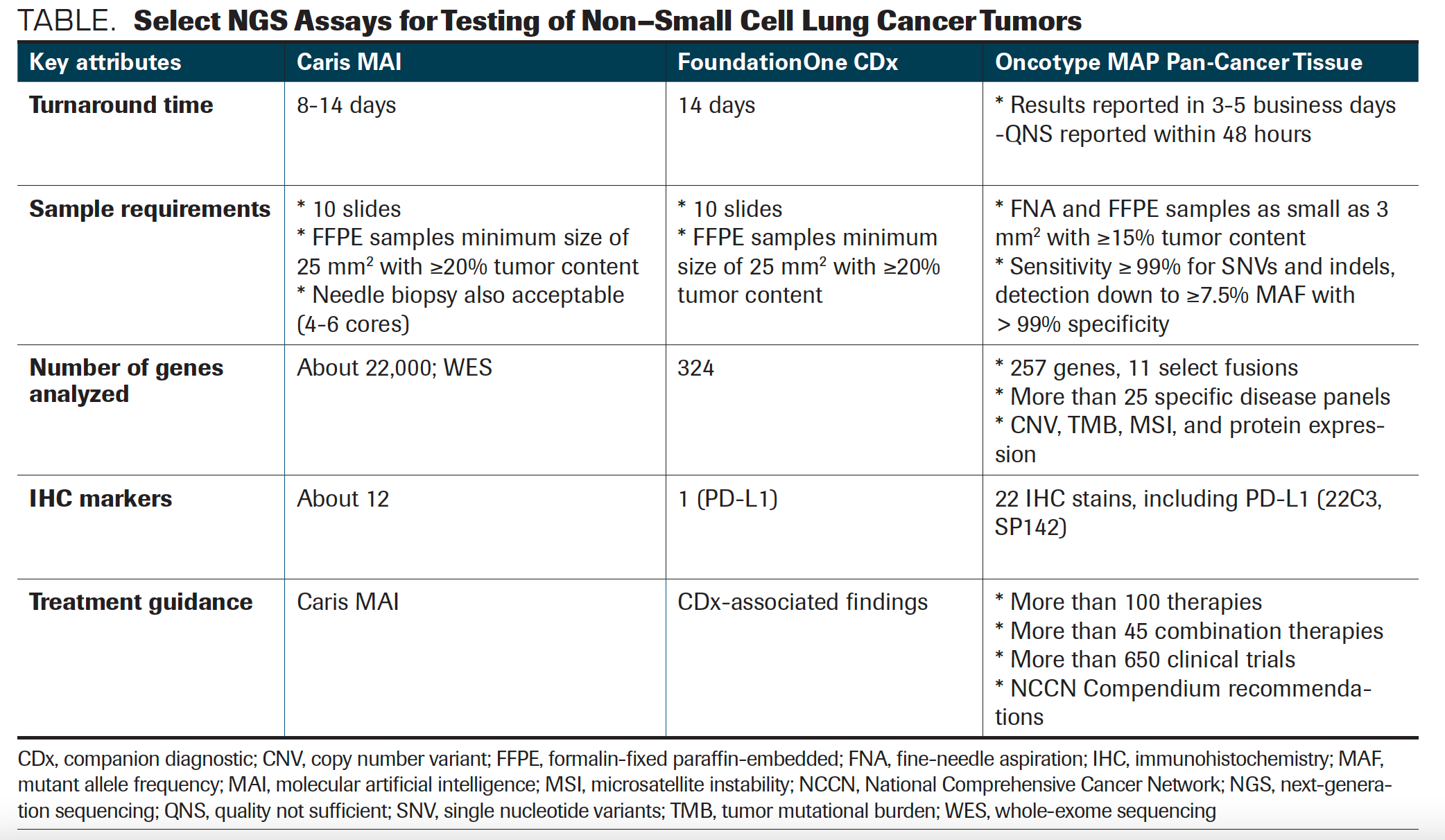
In discussing available NGS testing platforms for patients with metastatic NSCLC, Santos first reviewed Caris Molecular Intelligence Tumor Profiling, for which the turnaround time is between 8 and 14 days. The tissue sample requirement for this assay is 10 slides with a minimum size of 25 mm2 and a 20% or greater tumor content; this platform analyzes approximately 22,000 genes by whole-exome sequencing.
A second assay, FoundationOne CDx, has a turnaround time of 14 days, with tissue sampling requirements that are similar to the Caris test at 10 slides and a minimum size of 25 mm2 and 20% or greater tumor content. A total of 324 genes are analyzed and only 1 immunohistochemistry marker, PD-L1, is included; companion diagnostic tests are available to help guide treatment.
Finally, Santos discussed the Oncotype MAP Pan-Cancer Tissue Test, with a turnaround time of between 3 and 5 business days. “Quality not sufficient” results are reported more quickly, within 48 hours. Samples require tumor content of 15%; the test analyzes 257 genes as well as tumor mutational burden, microsatellite instability, and copy number variant. Twenty-two immunohistochemistry (IHC) markers are also included, by both IHC 223C and SP142 (TABLE).
TABLE. Select NGS Assays for Testing of Non–Small Cell Lung Cancer Tumors
Addressing Challenges With NGS Tumor Testing
Clinicians can face challenges with comprehensive genomic profiling, including obtaining tissue samples that meet minimum requirements and the tests’ long turnaround times. In addition, once profiling is done and molecular markers are identified, deciding upon the best treatment course can be no easy task.
The event participants shared their experiences regarding minimum tissue sampling requirements:
KRIS: [Meeting] minimum tissue sample requirements is among the biggest challenges [in comprehensive genomic profiling], because the problem is that either the tissue doesn’t exist or, as in my case because I work at a tertiary center, you are getting tissue from another institution, which is very hard. That is the number one problem. So, how do you deal with that? The only solution I can think of is to instill a culture of obtaining adequate tissue at the time of diagnosis, when lung cancer is first suspected. Even if we choose not to send it [immediately] for NGS testing, obtaining adequate tissue [is important to] make sure it’s in the pathology department, in case a decision is made at some point to send it for testing.
GANDARA: I agree that having the minimum sample size requirement was, in the past, a common problem. When we [talk to] community pathologists, twice yearly for lung cancer, we have a webinar discussing all the requirements for molecular testing. Every time a patient is entered on that trial, the pathologist fills out, at his institution, a form about the tumor purity: Are there any issues with the tissue specimen? Any crush artifacts or something else that would make it difficult to interpret? For most of these NGS panels, tumor purity has to be 20% or greater. From a clinician standpoint, the other thing that’s evolved very rapidly over the past 5 years, and this goes back to what Dr. Kris saying, is that in many situations we’re not accepting fine-needle aspiration. We’re having the pulmonologist or the radiologist do a core biopsy. We’re taking multiple needle passes to get the adequate amount of issue. Of course, if you have a block on the surgical biopsy or something similar, that’s going to be a lot easier for testing. I think this is getting better nationwide.
In discussing turnaround times and the implications for starting therapy, Kris shared best approaches for selecting treatment:
KRIS: When lung cancer is suspected, we are working hard to ensure that whoever does the biopsy does the ordering of the molecular tests. Some reflex testing is also done for some of the targets—specifically ALK, EGFR, and KRAS. NGS testing for us runs about 2 weeks, meaning it takes 2 weeks from when the specimen arrives in the pathology lab to get the results for the full NGS testing. [Results from] blood [arrive back] just a few days sooner than those from tissue.
It’s rare that you can’t wait until the result comes back, but there are some special situations where you have to start treatment before [then]. I think what happens is that if you start without the molecular results, it’s imperative that you continue whatever treatment you’ve started until the point where you can ascertain whether that treatment helped and what the tolerance is for therapy. Even if, for example, you found an EGFR mutation, if a patient has had a near-complete response and very good tolerance to therapy, I would be loath to change. [My takeaway is that] I would [encourage] everybody on the treatment team, when lung cancer is suspected, to try to get all of the molecular testing that you need.
Moving Forward
To wrap up the event, Gandara shared his thoughts regarding precision approaches in NSCLC. “[At this time,] there is no better tumor type for precision medicine than advanced NSCLC. We had 7 FDA approvals just in May [2020] alone, and 4 of them were genomic-based therapy,” he said.
“Test early, test often, and pay attention to the report for every scrap of information that can help you care for the patient,” concluded Kris.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
REFERENCES
1. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20(2):129-159. doi:10.1016/j.jmoldx.2017.11.004
2. Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491-1505. doi:10.1016/j.annonc.2020.07.014
3. NCCN. Clinical Practice Guidelines in Oncology. NSCLC cancer, version 3.2021. Accessed February 16, 2021. https://bit.ly/37JItWJ