Brain metastases are common in patients with non–small-cell lung cancer (NSCLC). Because of associated poor prognosis and limited specific treatment options, there is a real need for the development of medical therapies and strategies for affected patients. Novel compounds for epidermal growth factor receptor–dependent and anaplastic lymphoma kinase–dependent lung cancer have demonstrated blood-brain barrier permeability and have led to important improvements in central nervous system outcomes. Studies of targeted therapies for oncogene-driven tumors and of immunotherapies in patients with brain metastases have shown promise and, allied with novel radiation techniques, are driving a rapid evolution in treatment and prognosis for NSCLC brain metastases.
Introduction
Brain metastases are estimated to occur in 30% to 50% of patients with metastatic non–small-cell lung cancer (NSCLC).[1] Prognosis has historically been poor for these patients and is reported to be around 2 months with best supportive care.[2] Genomic characterization of lung tumors and matched targeted therapies have led to profound clinical benefit for patients, both in preventing or delaying the onset of brain metastases, and in leading to intracranial remissions for patients with preexisting lesions. Newer classes of compounds targeting the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), RET, MET, BRAF, NTRK, and ROS tyrosine kinases have varying blood-brain barrier (BBB) permeability. Current drug developmental strategies are aimed at increasing central nervous system (CNS) bioavailability by decreasing molecule size; increasing lipophilicity; and designing compounds that avoid common efflux proteins at the BBB, such as P-glycoproteins. Immuno-oncology also offers a novel pathway to clinical benefit, given the potential for antitumor immune effects at multiple organ sites, including the CNS. Here we discuss recent clinical investigations that highlight the effects of novel compounds targeting EGFR, ALK, and other receptor oncogenes, as well as the promise of immunotherapy in lung cancer CNS disease. We also update the current treatment paradigm for patients with lung cancer brain metastases, incorporating the latest developments.
Targeted Therapy
The CNS is a frequent site of NSCLC progression or diagnosis.[3,4] Rangachari and colleagues evaluated a cohort of 381 patients (86 EGFR-mutated and 23 ALK-rearranged) and found the incidence of brain metastases to be high, with CNS disease present at first diagnosis in 24.4% of EGFR-mutated and 23.8% of ALK-rearranged tumors.[5] The cumulative incidence of brain metastases at 3 years was 46.7% for EGFR-mutated and 58.4% for ALK-rearranged lung cancer. Targeting EGFR mutations and ALK rearrangements with small-molecule tyrosine kinase inhibitors (TKIs) has yielded high systemic response rates (RRs) in metastatic NSCLC and has improved progression-free survival (PFS) in the first-line setting. The development of targeted systemic approaches for treating brain metastases, allied to advances in radiation therapy, has the potential to reduce treatment-related toxicity in the CNS while prolonging life.
EGFR-Mediated Disease
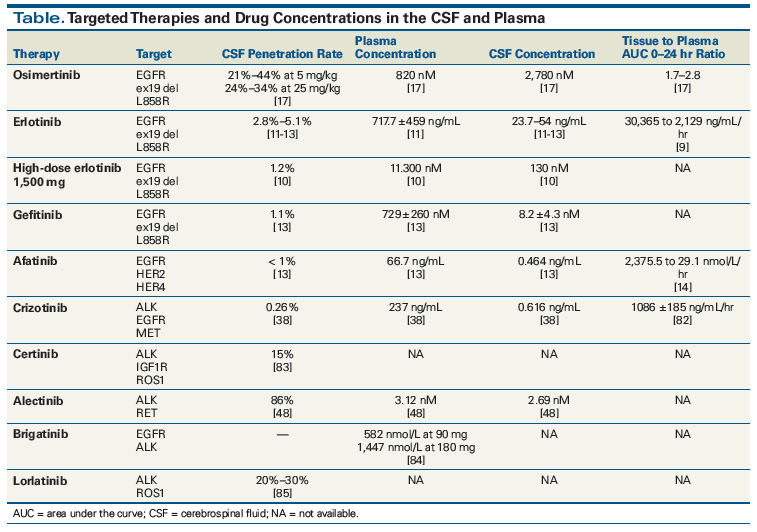
Between 10% and 20% of patients with lung adenocarcinoma are found to harbor activating EGFR mutations.[6] First- and second-generation EGFR TKIs (including erlotinib, gefitinib, and afatinib) are active in the CNS, with multiple retrospective studies showing brain RRs of over 50% in EGFR-mutated patients and prolonged survival times compared with EGFR wild-type patients.[7] Second site EGFR T790M mutations have been seen as a mechanism of resistance to first- and second-generation TKIs but are less common in brain metastases than in systemic metastases, likely because of lower drug penetration in the CNS and consequent lower drug exposure.[8] Cerebrospinal fluid (CSF) concentrations of erlotinib on standard 150-mg daily dosing are lower than systemic blood concentrations[9] because of this agent’s BBB permeability ratio of 2.8% to 5.1%.[10-12] Afatinib is an irreversible inhibitor of EGFR that has a brain penetration concentration of < 1%.[13] Data from 573 patients treated with afatinib, whose disease had previously progressed on erlotinib or gefitinib, showed a CNS RR of 35% (in 31 evaluable patients), with 66% achieving cerebral disease control.[14] Similarly, a case report documents that a patient who has CNS progression while receiving afatinib may respond to erlotinib.[15]
Osimertinib
The emergence of new anti-EGFR agents that can target resistant clones has resulted in further evolution of the landscape of targeted therapy against brain metastasis from primary NSCLC. One of these promising new compounds is osimertinib, a third-generation EGFR-mutant inhibitor that has been approved by the US Food and Drug Administration (FDA) for EGFR T790M–positive NSCLC.
Osimertinib showed preclinical evidence of concentrations in mouse brain tissue 5- to 25-fold higher than in plasma,[16] and has shown greater penetration of the BBB in mouse models than gefitinib, rociletinib, or afatinib.[17]An in vivo mouse leptomeningeal model demonstrated that osimertinib slowed the development of leptomeningeal carcinomatosis in both the treatment-naive and prior EGFR TKI–refractory settings.[18] Recent phase I data on osimertinib, 160 mg/d, in leptomeningeal disease of EGFR-mutant NSCLC (ClinicalTrials.gov identifier: NCT02228369) showed that 23 of 32 patients who underwent brain image assessment 12 weeks after initiation of treatment with osimertinib showed improvement: radiologic improvement in 10 patients and stable disease in 13 patients, and with 9 of the 23 patients demonstrating improvement in neurologic symptoms.[19] A 57% decrease in EGFR-mutant DNA copy was seen in 22 patients on cycle 2, day 1 CSF samples. Measurement of EGFR-mutant circulating tumor DNA (ctDNA) in the CSF showed that 7 of 9 patients had decreased ctDNA levels, with 5 patients having a > 50% decrease.[20] In the phase III AURA3 study, the median CNS PFS was significantly longer with osimertinib than with chemotherapy (11.7 vs 5.6 months; hazard ratio [HR], 0.32; P = .004). The CNS overall response rate (ORR) was 70% (21 of 30 patients) with osimertinib and 31% with chemotherapy.[21] In the recently presented FLAURA study, in which osimertinib was used in untreated patients with advanced EGFR-mutant NSCLC, the HR for systemic disease control and CNS control similarly favored osimertinib over erlotinib or gefitinib, supporting the preclinical data that showed osimertinib’s penetration across the BBB and providing support for using this agent in first-line management of EGFR-mutant patients with brain metastases.[22] Formal data on other compounds in development, including ASP8273, PF-7775, EGF816, and HM61713, in patients with brain metastases are still awaited.
Pulse dosing of first-generation EGFR inhibitors
In small, retrospective series, high-dose erlotinib was administered in a “pulsatile” fashion (1,500 mg weekly) in an attempt to achieve higher CSF drug concentrations and partial response in patients with leptomeningeal metastases from EGFR-mutant lung cancer. In one study, this approach led to a CNS partial response in 6 of 9 patients; however, in another study, only 1 of 10 patients experienced a partial response with the same strategy, and a dismal 1.7-month median overall survival (OS) was reported.[23,24] Results similar to the latter were seen in another study of patients with leptomeningeal disease, with high-dose EGFR TKIs leading to an objective response in 3 of 10 patients (30%).[25] In a phase I dose-escalation study of pulsed erlotinib in the front-line treatment of 34 patients with metastatic disease (ClinicalTrials.gov identifier: NCT01967095), the maximum tolerated dose was found to be 1,200 mg on days 1 and 2 and 50 mg on days 3 through 7 weekly; no patients withdrew from the study because of CNS metastases, and 22 of 27 evaluable patients (81%) had a partial response systemically.[26] To date, there is no trial systematically randomizing patients between pulsatile dosing and standard dosing, and in most studies of the pulsatile approach, this has been evaluated in previously treated patients whose disease has progressed on standard-dose first-line EGFR TKI therapy. Pulsatile dosing is presumed to increase intracranial drug distribution; however, it will not likely improve outcomes when progression is driven by a molecular pathway that renders the tumor unresponsive to the agent under investigation.
Additional strategies in EGFR-mutant patients
Preclinical data have demonstrated that radiation increases EGFR expression and that blocking EGFR in vitro sensitizes cells to radiation.[27,28] In an unselected phase II study, concomitant whole-brain radiation therapy (WBRT) and standard-dose erlotinib were well tolerated, with an ORR of 86%.[29] The Radiation Therapy Oncology Group study 0320 evaluated WBRT + stereotactic radiosurgery (SRS) + erlotinib and found significant grade 3–5 toxicity rates of 49%, compared with a rate of 11% for WBRT alone; the toxicities observed included cytopenias, rash, fatigue, and dehydration, among others.[30]
Efforts have been underway to develop EGFR inhibitors that can specifically penetrate the BBB. Studies of a novel compound, AZD3759, showed that it has high passive permeability of the BBB and is not affected by efflux transporters.[31] Animal brain metastasis models have demonstrated significant tumor response and improved survival with this compound.[32] In patients with brain metastases in the AZD3759 phase I clinical trial, 63% (12 of 19 patients) achieved an intracranial response and 50% (10 of 20 patients) had an extracranial response.[33] It remains unclear how the BBB permeability of AZD3759 will compare with that of osimertinib.
ALK-Translocated Disease
ALK gene fusions have been observed in 2% to 7% of patients with NSCLC in reported series.[34] Despite high systemic RRs, many patients exhibit disease progression with CNS metastasis within 1 year of starting crizotinib therapy. Several prospective studies show a brain metastasis prevalence of 40% to 70% in patients previously treated with ALK inhibitors.[35] It remains unclear whether this increased prevalence is entirely related to inadequate pharmacologic penetration or whether a more aggressive biology, with more and earlier brain metastatic disease, is also a factor. Measurements of the CSF concentration of crizotinib while patients are receiving therapy are quite low, with one report of a CSF-to-serum ratio of < 0.1%[36] and another report of a ratio of 0.26%.[37] Despite the low CSF penetration of crizotinib, analysis of the phase III PROFILE 1005 and 1007 trials of crizotinib showed efficacy, with CNS disease control rates (DCR) comparable to systemic DCR (about 55% at 12 weeks) and 18% to 33% of patients demonstrating CNS response.[38] The median time to CNS progression was 7 months in patients with previously untreated brain metastases.[38] A retrospective study of 90 patients with ALK-rearranged NSCLC metastatic to the brain found that OS after the development of brain metastases was still prolonged at approximately 4 years (49.5 months), with patients frequently having multiple opportunities for salvage SRS at recurrence; 45% of patients on follow-up had progressive brain metastases at death.[39] More than 50% of patients treated with radiotherapy underwent a repeat course of therapy, and 25% underwent three or more interventions with radiation. Absence of extracranial metastases, Karnofsky Performance Status score ≥ 90, and no history of TKI therapy before the development of brain metastases were associated with improved survival.[39] These results underscore the importance of monitoring long-term treatment of CNS metastasis in this subtype of lung cancer, and the importance of focusing on non–WBRT-based treatments to achieve a meaningful response.[38,39]
Ongoing studies are currently investigating the inhibition of P-glycoprotein together with crizotinib to increase drug concentrations in the CSF.[40] In mouse models, the concurrent administration of the compound elacridar (which inhibits P-glycoprotein) along with crizotinib enhanced the intracranial accumulation of crizotinib at 24 hours 70-fold. To improve outcomes, some reports include use of high-dose crizotinib[41] or high-dose pemetrexed with high-dose crizotinib.[40]
Ceritinib
Second-generation ALK inhibitors have shown promising CNS efficacy. The phase I ASCEND-1 trial of ceritinib evaluated 124 patients with brain metastases and showed a complete or partial response in 7 of 14 patients (50%) with Response Evaluation Criteria in Solid Tumors (RECIST)-evaluable brain metastases.[42] In the phase II ASCEND-2 trial, the CNS DCR was 80.0% in 5 of 6 patients with brain lesions. Nausea, diarrhea, and vomiting were reported as the most common adverse events.[43] Another phase II study (ASCEND-3) included 10 patients with investigator-assessed metastatic brain lesions at baseline and reported a 20% CNS RR and an 80% intracranial DCR.[44] A phase II study of ceritinib for ALK-rearranged intracranial metastatic disease and leptomeningeal disease (ASCEND-7; ClinicalTrials.gov identifier: NCT02336451) is in progress; in this study, CSF samples will be assessed to further investigate the intracranial penetration of this drug.
KEY POINTS
- Novel strategies that are effective in treating brain metastases in patients with lung cancer are needed.
- Patients with brain metastases who have oncogenic drivers, including EGFR and ALK, may receive next-generation targeted therapy that is effective against central nervous system disease.
- Patients being treated with immunotherapy may have an intracranial response while receiving therapy.
Alectinib
Preclinical studies of the pharmacokinetics of alectinib have shown substantial improvement in CNS penetration of the drug compared with crizotinib; the concentration of alectinib in the CNS is reported to be 63% to 94% of that measured in the serum.[45] These high CNS concentrations might be accounted for by the fact that alectinib, unlike crizotinib and ceritinib, is not a substrate for P-glycoprotein and is therefore not actively expelled from the intracranial environment.[8] Pooled efficacy and safety data from two single-arm phase II studies (ClinicalTrials.gov identifiers: NCT01871805 and NCT01801111), showed that in 163 patients with baseline CNS metastases, the CNS ORR was 64.0%, the CNS DCR was 90.0%, and the median duration of response was 10.8 months after a median follow-up of 12.4 months.[8] The ORR was 35.8% for patients with prior radiotherapy exposure and 58.5% for those who had not received prior radiotherapy.[8] Findings from the ALUR trial,[46] as well as a secondary analysis of the ALEX trial,[47] and an earlier AF-002JG study[48] showed decreased CNS progression of NSCLC with alectinib in the first- and second-line treatment settings. ALUR included 107 ALK rearrangement–positive NSCLC patients whose disease had progressed after treatment with platinum-based chemotherapy and crizotinib.[49] Among patients who had measurable CNS disease at baseline, the CNS ORR was 54.2% in those treated with alectinib, compared with 0% in the chemotherapy arm (P < .001). The 6-month cumulative incidence rate of CNS progressive disease was 11% for alectinib vs 48% for chemotherapy. PFS was significantly longer in the alectinib group compared with the chemotherapy group (9.6 vs 1.4 months, respectively; HR, 0.15; P < .001). A marked difference in CNS DCR was observed (80.6% for alectinib vs 28.6% for chemotherapy), with improvement in neurocognitive testing.
In the ALEX first-line study of alectinib vs crizotinib, a subgroup analysis showed that CNS progression occurred in 12% of patients in the alectinib group vs 45% of those in the crizotinib group (HR, 0.16; P < .001).[47] CNS response occurred in 17 of 21 patients receiving alectinib (81%), and the median duration of intracranial response was 17.3 months in the alectinib group vs 5.5 months in the crizotinib group. These results were consistent with those of the previously reported J-ALEX study, which was a similarly designed randomized phase III trial (alectinib vs crizotinib).[50] However, only 13.6% of patients had measurable brain lesions in the alectinib arm of J-ALEX compared with 42% in the ALEX trial. In J-ALEX, the 300-mg twice-daily alectinib dose used demonstrated CNS RRs comparable to those achieved with a 600-mg twice-daily dose in ALEX. In the subgroup of patients with brain metastases in J-ALEX, a strikingly improved response to alectinib (HR, 0.08) compared with crizotinib was also observed in patients with brain metastases at baseline.
Brigatinib
In an initial phase I/II trial, brigatinib demonstrated an intracranial ORR of 50% in patients with baseline CNS metastases and a median intracranial PFS of nearly 2 years.[51] In an ongoing study (ClinicalTrials.gov identifier: NCT02094573), 4 of 5 patients showed intracranial response to brigatinib.[52] In an update of the phase II ALTA trial, brigatinib continued to show robust intracranial efficacy in ALK rearrangement–positive NSCLC patients with baseline brain metastases. With regard to the intracranial response of patients with measurable brain metastases, results showed a median duration of response of 16.6 months, and patients randomized to lead-in with brigatinib at 90 mg/d followed by 180-mg/d dosing demonstrated greater ORR compared with patients who received 90 mg/d (67% and 50%, respectively).[53]
Lorlatinib
Lorlatinib is a CNS-penetrating compound and has potent activity against de novo fusions and kinase domain resistance mutations.[54] In the phase I component of an ongoing phase I/II study (ClinicalTrials.gov identifier: NCT01970865), patients with ALK rearrangement–positive or ROS1 rearrangement–positive NSCLC with or without brain metastases had confirmed intracranial ORRs of 75% in treatment-naive patients (6 of 8 patients) and 15% to 25% in those who had received 2 or 3 lines of prior treatment.[55] Lorlatinib was well tolerated and demonstrated durable intracranial responses in ALK-rearranged NSCLC patients (including those with G1202R resistance mutations) and ROS1-rearranged patients, many of whom had CNS metastases and had received one or more prior TKI. The FDA has granted a Breakthrough Therapy designation to lorlatinib for use in patients with ALK rearrangement–positive metastatic NSCLC who have previously received up to three prior ALK TKIs. In a phase II study of lorlatinib in 23 patients with target lesions, the intracranial ORR was 47%, with 7 patients (30%) demonstrating a complete response, 4 patients (17%) demonstrating partial response, and 2 with unconfirmed partial response. The median duration of response was 10.5 months among ALK rearrangement–positive patients and 12.4 months among ALK rearrangement–positive/ROS1 rearrangement–positive patients.[54,55]
A practical approach to ALK therapy in patients with brain metastases
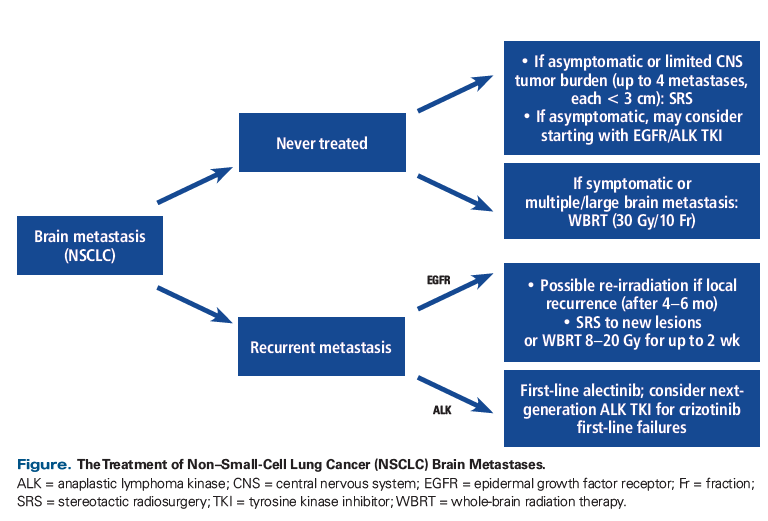
The National Comprehensive Cancer Network recommends that NSCLC patients who present with asymptomatic brain metastases be started on ALK inhibitor therapy alone.[56] Because data on the adverse effects of combining radiation with crizotinib for intracranial disease are scarce, the drug should be discontinued at least a day before starting radiation therapy; it can be restarted afterwards.[57] Symptomatic patients should be considered for surgery ± SRS or WBRT, followed by an ALK inhibitor. If the disease burden is low (up to 10 metastases), SRS should be recommended to avoid the adverse cognitive effects of WBRT.[58] Given the frequency of CNS metastasis, ALK rearrangement–positive patients might also benefit from MRI surveillance with shorter intervals.[59] If patients progress through the available ALK inhibitors and radiation, conventional treatment with pemetrexed is a viable option for ALK-rearranged intracranial disease. In some cases, pemetrexed has demonstrated meaningful activity in brain metastasis or CNS disease, despite low penetration into the CNS (5%).[60] Retrospective studies of patients with unselected histology reported an intracranial RR for pemetrexed of 30.8%, with clinical benefit of around 70%.[61] A study of 36 patients with metastatic NSCLC receiving cisplatin, 75 mg/m2, and pemetrexed, 500 mg/m2, every 3 weeks showed intracranial and extracranial RRs of 42% and 35%, respectively, with a 7.4-month median survival; these results demonstrate that the addition of platinum compounds to pemetrexed can improve outcomes in patients with brain metastases.[62]
Leptomeningeal Disease in Patients With Wild-Type NSCLC
Intrathecal (IT) chemotherapy has been evaluated in patients with NSCLC leptomeningeal carcinomatosis; the most commonly used agents have been methotrexate, cytarabine, and thiotepa. The prognosis for solid tumor leptomeningeal disease is generally very poor, and the effectiveness of IT chemotherapy for NSCLC is questionable. An early study randomizing 59 patients (12 with lung cancer) to IT methotrexate vs IT thiotepa found that, although there was clearance of CSF cytology in some patients (in lung cancer specifically, in 4 out of 12 patients), there was no significant clinical neurologic improvement, and the median survival was only about 15 weeks.[63] Another study that evaluated 26 patients (17 with lung cancer) treated with a combination of IT methotrexate, cytarabine, and hydrocortisone showed a cytologic RR of 38.5% but a median survival of only 18.6 weeks.[64] Of note, depot cytarabine has been permanently discontinued by the manufacturer; thus, only the short-acting formulation will be available. High-dose methotrexate (3–8 g/m2) rather than IT chemotherapy may be considered for patients with wild-type CNS metastases in order to achieve CNS cytologic clearance and cytotoxic CSF methotrexate levels. However, limited objective antitumor responses have been seen with this approach.[65]
Immunotherapy
Immune checkpoint inhibitors are currently approved for use in many malignancies that may metastasize to the brain, including melanoma, renal cell carcinoma, and NSCLC (squamous and adenocarcinoma). The density of tumor-infiltrating lymphocytes in brain metastases has showed a correlation with OS in patients across tumors.[66] In the phase II trial of nivolumab in squamous NSCLC (CheckMate 063), 4 patients with brain metastases were enrolled, and of the 2 that were evaluable, both had responses in their CNS lesions.[67] CheckMate 012 (ClinicalTrials.gov identifier: NCT01454102) is an ongoing trial of nivolumab in advanced NSCLC that contains an arm evaluating safety and tolerability in patients with untreated, asymptomatic brain metastases.
Some of the initial studies investigating the effect of checkpoint inhibitors on CNS metastases were conducted in patients with melanoma, including a retrospective analysis of the phase II trial of ipilimumab, which showed intracranial responses in 5 of 12 patients with baseline CNS metastases.[68] Additional phase II data have suggested a 20% 1-year survival rate in such patients.[69] Preliminary clinical data on pembrolizumab in the setting of metastatic NSCLC showed that 4 of 9 evaluable patients had partial responses (44%), and that 3 of the 9 patients had progressive disease (34%) as the initial response of their brain metastases; in 22% of patients, the brain was unevaluable because of rapid systemic disease progression.[70] A phase II trial of pembrolizumab in patients with metastatic melanoma or NSCLC and untreated brain metastases demonstrated a strong concordance between the responses in brain and extracerebral metastases.[71] In a retrospective evaluation of 5 patients with advanced NSCLC and new or progressing asymptomatic intracranial metastases on nivolumab, one complete and one partial intracranial response were seen. Stabilization of leptomeningeal carcinomatosis for 10 weeks was achieved in 1 additional patient, while 2 patients showed CNS progression. Time to response was 5 and 9 weeks, respectively, in the 2 patients with partial responses, and both responses were still ongoing at 24+ and 28+ weeks, respectively, since the start of treatment; systemic responses and intracranial responses were largely concordant.[72]
Treating brain metastases in NSCLC with checkpoint inhibitor therapy can be confounded by the effects of using high-dose corticosteroids in this patient population. Some clinicians are moving away from using high-dose steroids for patients with brain metastases who are treated with checkpoint inhibitors, although data suggest that steroids administered for ipilimumab-induced immune-related adverse events do not adversely impact the efficacy of treatment, and further evaluation is thus warranted.[69,70] Checkpoint inhibitor therapy–related toxicities such as perilesional edema and necrosis in previously irradiated lesions and intralesional hemorrhage warrant careful consideration.[73] A deeper understanding of the highly regulated immune microenvironment of the brain should provide some answers in the future. A phase II study of pembrolizumab (ClinicalTrials.gov identifier: NCT03091478) in patients with advanced solid tumors and leptomeningeal carcinomatosis is currently ongoing using pembrolizumab at a dose of 200 mg IV every 3 weeks for 4 doses to evaluate response, while allowing nonescalating doses of steroids in order to facilitate local radiation therapy.
Combinations of Radiation and Immunotherapy
Evidence suggests that responses may be further improved by combining immune checkpoint inhibitors with radiation.[74] An abscopal effect, in which local radiation may lead to a distant systemic response, may be achieved by combining checkpoint inhibitor and radiation therapy. Fractionated radiation to the primary site may produce an abscopal effect with response at a secondary site when combined with checkpoint inhibitor therapy.[75] Cell death may generate an immune response via enhancement of major histocompatibility complex class I expression, specific antigen processing, and CD8+ T-cell activation.[76] Radiation upregulates inflammatory cytokines (ie, tumor necrosis factor α, interferon γ, and chemokine [C-X-C motif] ligand 16), promoting tumor detection and facilitating T-cell infiltration.[77] Radiation can also upregulate programmed death ligand 1 (PD-L1), which may lead to T-cell exhaustion and enhance the diversity of the T-cell receptor repertoire of intratumoral T cells.[78] Anti–cytotoxic T-lymphocyte–associated antigen 4 promotes expansion of T cells, while radiation may have effects that shape the T-cell receptor repertoire. Adding PD-L1 inhibitors reverses T-cell exhaustion and further encourages T-cell expansion.[78,79] The abscopal effect supports the use of radiation combined with immune-modulating agents by increasing antibody responses to other antigens after radiotherapy.[80] In a melanoma case series that included patients with brain metastases, a durable response with a median survival of 21.3 months was achieved with ipilimumab after SRS, compared with a median survival of only 4.9 months with SRS alone.[74] Perilesional edema seen on fluid attenuation inversion recovery (FLAIR) images before and during therapy in patients with brain metastases receiving checkpoint inhibitors following SRS may suggest immune response. However, with current imaging modalities, it is difficult to determine whether lesions have enlarged with immune checkpoint inhibitor treatment that follows SRS because of inflammation, necrosis, or tumor growth.[81]
Conclusion
The combination of new drugs and new technologies is changing the way we characterize and treat brain metastases in patients with lung cancer. Further studies are underway to explore carefully the effects of new therapies, using pre- and post-treatment tissue biopsies and careful analysis of the CSF. Ongoing studies of targeted therapies and immunotherapies have the potential to have a significant positive impact on the clinical management of patients with brain metastases.
Financial Disclosure:Dr. Bulbul serves on advisory boards for AstraZeneca and Pfizer. Dr. Forde serves as a consultant to AstraZeneca, Bristol-Myers Squibb, EMD Serono, and Novartis. Dr. Ettinger serves as an advisor to Bristol-Myers Squibb, Eli Lilly & Co, and Genentech. Dr. Husain serves on the advisory board for AstraZeneca; he serves on speakers’ bureaus for and receives honoraria from AstraZeneca, Bristol-Myers Squibb, and Merck. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166.
2. Chang DB. Late survival of non-small cell lung cancer patients with brain metastases: influence of treatment. CHEST J. 1992;101:1293-7.
3. Motl S, Zhuang Y, Waters CM, Stewart CF. Pharmacokinetic considerations in the treatment of CNS tumours. Clin Pharmacokinet. 2006;45:871-903.
4. Gainor JF, Ou SH, Logan J, et al. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8:1570-3.
5. Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108-11.
6. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306-11.
7. Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40:716-22.
8. Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:4079-85.
9. Broniscer A, Panetta JC, O’Shaughnessy M, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 2017;13:1511-5.
10. Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol. 2014;2:116-20.
11. Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5:950-5.
12. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399-405.
13. Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10:156-63.
14. Zhang SR, Zhu LC, Jiang YP, et al. Efficacy of afatinib, an irreversible ErbB family blocker, in the treatment of intracerebral metastases of non-small cell lung cancer in mice. Acta Pharmacologica Sinica. 2017;38:233-40.
15. Nonagase Y, Okamoto K, Iwasa T, et al. Afatinib-refractory brain metastases from EGFR-mutant non-small-cell lung cancer successfully controlled with erlotinib: a case report. Anticancer Drugs. 2016;27:251-3.
16. Kim DW, Yang JC, Cross D, et al. Preclinical evidence and clinical cases of AZD9291 activity in EGFR-mutant non-small cell lung cancer (NSCLC) brain metastases (BM). Ann Oncol. 2014;25(suppl 4):iv146-iv164.
17. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130-40.
18. Nanjo S, Ebi H, Arai S, et al. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget. 2016;7:3847-56.
19. Yang JC, Cho B, Kim DW, et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): updated results from BLOOM study. J Clin Oncol. 2017;35(suppl):abstr 2020.
20. Lee DH, Kim DW, Ahn MJ, et al. AZD9291 activity in patients with leptomeningeal disease from non-small cell lung cancer: a phase I study. Presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; Nov 5–9, 2015; Boston, MA. Abstr PR07.
21. Mok T, Ahn MJ, Han JY, et al. CNS response to osimertinib in patients (pts) with T790M-positive advanced NSCLC: data from a randomized phase III trial (AURA3). J Clin Oncol. 2017;35(15_suppl):abstr 9005.
22. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113-25.
23. Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364-9.
24. Jackman DM, Mach SL, Heng JC, et al. Pulsed dosing of erlotinib for central nervous system (CNS) progression in EGFR-mutant non-small cell lung cancer (NSCLC). J Clin Oncol. 2013;31(15_suppl):abstr 8116.
25. Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75:1261-6.
26. Yu HA, Sima CS, Reales D, et al. A phase I study of twice weekly pulse dose and daily low dose erlotinib as initial treatment for patients (pts) with EGFR-mutant lung cancers. J Clin Oncol. 2015;33(15 suppl):abstr 8017.
27. Akimoto T, Hunter NR, Buchmiller L, et al. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5:2884-90.
28. Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65:3328-35.
29. Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895-902.
30. Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312-8.
31. Kim D, Yang JC, Chen K, et al. AZD3759, an EGFR inhibitor with blood brain barrier (BBB) penetration for the treatment of non-small cell lung cancer (NSCLC) with brain metastasis (BM): preclinical evidence and clinical cases. J Clin Oncol. 2015;33(suppl):abstr 8016.
32. Yang Z, Guo Q, Wang Y, et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med. 2016;8:368ra172.
33. Ahn MJ, Kim DW, Cho BC, et al. Phase I study (BLOOM) of AZD3759, a BBB penetrable EGFR inhibitor, in patients with TKI-naïve, EGFRm NSCLC with CNS metastases. J Clin Oncol. 2017;35(15_suppl):abstr 2006.
34. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247-53.
35. Toyokawa G, Seto T, Takenoyama M, Ichinose Y. Insights into brain metastasis in patients with ALK+ lung cancer: is the brain truly a sanctuary? Cancer Metastasis Rev. 2015;34:797-805.
36. Metro G, Lunardi G, Floridi P, et al. CSF concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10:e26-e27.
37. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443-e445.
38. Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881-8.
39. Johung KL, Yeh N, Desai NB, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2015;34:123-9.
40. Gandhi L, Drappatz J, Ramaiya NH, Otterson GA. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J Thorac Oncol. 2013;8:e3-e5.
41. Kim YH, Ozasa H, Nagai H, et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J Thorac Oncol. 2013;8:e85-e86.
42. Shaw A, Mehra R, Tan DS, et al. BM-32: Ceritinib (LDK378) for treatment of patients with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) and brain metastases (BM) in the ASCEND-1 trial. Neuro Oncol. 2014;16(suppl 5):v39.
43. Mok T, Spigel D, Felip E, et al. ASCEND-2: a single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ). J Clin Oncol. 2015;33(15_suppl):abstr 8059.
44. Park K, Tan D, Ahn MJ, et al. 419O: Efficacy and safety of ceritinib in patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) and baseline brain metastases (BM)-results from ASCEND-2 and ASCEND-3. Ann Oncol. 2015;26(suppl_9):ix126-ix127.
45. Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023-8.
46. Wolf J, Oh IJ, Mazieres J, et al. ALUR: a phase 3 study of alectinib versus chemotherapy in previously treated ALK+ non-small cell lung cancer (NSCLC). Ann Oncol. 2016;27(suppl_6):1290TiP.
47. Gadgeel S, Peters S, Mok T, et al. Alectinib vs crizotinib in treatment-naïve ALK+ NSCLC: CNS efficacy results from the ALEX study. Ann Oncol. 2017;28(suppl_5):v605-v649.
48. Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119-28.
49. de Castro J, Novello S, Mazieres J, et al. CNS efficacy results from the phase III ALUR study of alectinib vs chemotherapy in previously treated ALK+ NSCLC. Ann Oncol. 2017;28(suppl_5):abstr 1346P.
50. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29-39.
51. Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(15_suppl):abstr 8062.
52. Ou SH, Tiseo M, Camidge R, et al. Intracranial efficacy of brigatinib (BRG) in patients (Pts) with crizotinib (CRZ)-refractory anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) and baseline CNS metastases. Ann Oncol. 2017;28(suppl_5):abstr 1345P.
53. Ahn M, Camidge DR, Tiseo M, et al. OA.05.05: Brigatinib in crizotinib-refractory ALK+ NSCLC: updated efficacy and safety results from ALTA, a randomized phase 2 trial. J Thorac Oncol. 2017;12(Suppl 2):S1755-S1756.
54. Solomon B, Shaw A, Ou S, et al. OA 05.06: Phase 2 study of lorlatinib in patients with advanced ALK+/ROS1+ non-small-cell lung cancer. J Thorac Oncol. 2017;12:S1756.
55. Felip E, Bauer T, Solomon B, et al. MA07.11: Safety and efficacy of lorlatinib (PF-06463922) in patients with advanced ALK+ or ROS1+ non-small-cell lung cancer (NSCLC). J Thorac Oncol. 2017;12:S383-S384.
56. The National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: non small cell lung cancer. Version 4.2016. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl. Accessed March 14, 2018.
57. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-95.
58. Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61:185-91.
59. Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16:e510-e521.
60. Kumthekar P, Grimm SA, Avram MJ, et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neurooncol. 2013;112:247-55.
61. Bearz A, Garassino I, Tiseo M, et al. Activity of pemetrexed on brain metastases from non-small cell lung cancer. Lung Cancer. 2010;68:264-8.
62. Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. 2011;22:2466-70.
63. Grossman SA, Finkelstein DM, Ruckdeschel JC, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11:561-9.
64. Kim DY, Lee KW, Yun T, et al. Comparison of intrathecal chemotherapy for leptomeningeal carcinomatosis of a solid tumor: methotrexate alone versus methotrexate in combination with cytosine arabinoside and hydrocortisone. Jpn J Clin Oncol. 2003;33:608-12.
65. Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561-7.
66. Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2015;5:e1057388.
67. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257-65.
68. Weber JS, Amin A, Minor D, et al. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21:530-4.
69. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459-65.
70. Goldberg SB, Gettinger SN, Mahajan A, et al. Activity and safety of pembrolizumab in patients with metastatic non-small cell lung cancer with untreated brain metastases. J Clin Oncol. 2015;33(15-suppl):abstr 8035.
71. Kluger HM, Goldberg SB, Sznol M, et al. Safety and activity of pembrolizumab in melanoma patients with untreated brain metastases. J Clin Oncol. 2015;33(15_suppl):9009.
72. Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer. 2016;98:114-7.
73. Cohen JV, Kluger HM. Systemic immunotherapy for the treatment of brain metastases. Front Oncol. 2016;6:49.
74. Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117:227-33.
75. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379-88.
76. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259-71.
77. Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29-36.
78. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373-7.
79. Berghoff AS, Preusser M. The inflammatory microenvironment in brain metastases: potential treatment target? Chin Clin Oncol. 2015;4:21.
80. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925-31.
81. Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125:17-23.
82. Tang SC, Nguyen LN, Sparidans RW, et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer. 2014;134:1484-94.
83. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682-9.
84. Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first-and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22:5527-38.
85. Zou HY, Engstrom LR, Li Q, et al. PF-06463922, a novel brain-penetrating small molecule inhibitor of ALK/ROS1 with potent activity against a broad spectrum of ALK resistant mutations in preclinical models in vitro and in vivo. Mol Cancer Ther. 2013;11(Suppl):abstr C253.