Talabostat Active in Phase II Trials in Stage IV Melanoma, CLL
Talabostat (PT-100, Point Therapeutics), an oral, small-molecule inhibitor of dipeptidyl peptidase (DPP) fast-tracked by the FDA for stage IIIB/IV non-small-cell lung cancer, also looks promising in salvage regimens for patients with advanced melanoma or chronic lymphocytic leukemia (CLL
ATLANTATalabostat (PT-100, Point Therapeutics), an oral, small-molecule inhibitor of dipeptidyl peptidase (DPP) fast-tracked by the FDA for stage IIIB/IV non-small-cell lung cancer, also looks promising in salvage regimens for patients with advanced melanoma or chronic lymphocytic leukemia (CLL), according to posters presented at the American Society of Clinical Oncology 42nd Annual Meeting
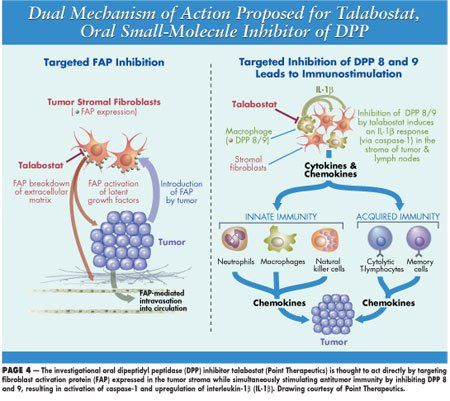
Talabostat inhibits DPPs such as fibroblast activation protein (FAP), which is found on the stroma of tumors, draining lymph nodes and in melanomas, and CD26, which is abnormally expressed in some types of CLL. The agent also upregulates cytokines and chemokines, which increases both innate and acquired T-cell immunity (see proposed mechanism of action drawing on page 1). Casey Cunningham, MD, of Mary Crowley Medical Research Center, Dallas, reported results of an open-label, single-arm, phase II study of talabostat and cisplatin in stage IV melanoma (abstract 8040). Patients received four 3-week cycles of cisplatin (75 mg/m2 on day 1) and talabostat (300 µg twice daily orally on days 2 to 15) with dose-escalation to talabostat 400 µg twice daily depending on tolerability. Patients continued single-agent talabostat until disease progression or unacceptable toxicity.
The study enrolled 74 patients with histologically or cytologically confirmed unresectable stage IV metastatic melanoma and no more than one prior chemotherapy or biotherapy for stage IV disease. "Forty-two percent of patients were considered unevaluable, primarily due to high-dose cisplatin-associated nausea and vomiting, limiting evaluation of its activity in combination with chemotherapy," he said.
Partial responses were seen in 6 of 43 (13.9%) evaluable patients. Response duration ranged from 4 to 10 months. Twenty more patients (46.5%) had stable disease for four or more cycles. Estimated median progression-free survival (PFS) was 2.8 months; estimated overall survival was 8.5 months. "Additional studies of talabostat in melanoma with other drug or biologic combinations are warranted," Dr. Cunningham concluded.
Rituximab-Resistant CLL
Khuda D. Khan, MD, PhD, director of cancer research, St. Francis Hospial, Indianapolis, reported results of an open-label phase II study of talabostat and rituximab (Rituxan) in patients with fludarabine/rituximab-resistant or refractory CLL (abstract 6598). They received rituximab 375 mg/m2 weekly for 4 weeks, and talabostat 300 µg twice daily for 6 days after each rituximab infusion.
Dr. Khan reported partial responses in 8 of 42 evaluable patients (19%). Of the 8 responders, 6 had failed prior rituximab and three of these had also failed alemtuzumab (Campath). Response duration ranges from 2 to more than 11 months. Estimated PFS was 3.6 months in all patients, 2.9 months in patients receiving prior rituximab, and 5.1 months in the alemtuzumab failures.
"Talabostat was shown to enhance the activity of rituximab in patients with rituximab-refractory or relapsed B-CLL malignancies, most likely by enhancing the antibody-dependent cytotoxicity of rituximab," Dr. Khan said. "Additional studies are warranted."