Targeted Therapy in Rectal Cancer
Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) are often overexpressed in colorectal cancer and are associated with inferior outcomes. Based on successful randomized phase III trials, anti-EGFR and anti-VEGF therapeutics have entered clinical practice. Cetuximab (Erbitux), an EGFR-specific antibody, is currently approved in the United States in combination with irinotecan (Camptosar) for patients with metastatic colorectal cancer refractory to irinotecan or as a single agent for patients unable to tolerate irinotecan-based therapy. In retrospective analyses, patients with EGFR-expressing rectal cancer undergoing neoadjuvant radiation therapy had a significantly inferior disease-free survival and lower rates of achieving pathologic complete response. Based on the positive data in metastatic colorectal cancer and synergy with radiation therapy seen in preclinical models, there is a strong rationale to combine cetuximab with neoadjuvant radiation therapy and chemotherapy in rectal cancer. Bevacizumab (Avastin), a VEGF-specific antibody, was the first antiangiogenic agent to be approved in the United States for use in combination with standard chemotherapy in the first- and second-line of treatment in metastatic colorectal cancer. VEGF-targeted therapy may lead to indirect killing of cancer cells by damaging tumor blood vessels, and may increase the radiosensitivity of tumor-associated endothelial cells. VEGF blockade can also "normalize" tumor vasculature, thereby leading to greater tumor oxygenation and drug penetration. This review will address completed and ongoing trials that have established and continue to clarify the effects of these agents in rectal cancer.
ABSTRACT: ABSTRACT: Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) are often overexpressed in colorectal cancer and are associated with inferior outcomes. Based on successful randomized phase III trials, anti-EGFR and anti-VEGF therapeutics have entered clinical practice. Cetuximab (Erbitux), an EGFR-specific antibody, is currently approved in the United States in combination with irinotecan (Camptosar) for patients with metastatic colorectal cancer refractory to irinotecan or as a single agent for patients unable to tolerate irinotecan-based therapy. In retrospective analyses, patients with EGFR-expressing rectal cancer undergoing neoadjuvant radiation therapy had a significantly inferior disease-free survival and lower rates of achieving pathologic complete response. Based on the positive data in metastatic colorectal cancer and synergy with radiation therapy seen in preclinical models, there is a strong rationale to combine cetuximab with neoadjuvant radiation therapy and chemotherapy in rectal cancer. Bevacizumab (Avastin), a VEGF-specific antibody, was the first antiangiogenic agent to be approved in the United States for use in combination with standard chemotherapy in the first- and second-line of treatment in metastatic colorectal cancer. VEGF-targeted therapy may lead to indirect killing of cancer cells by damaging tumor blood vessels, and may increase the radiosensitivity of tumor-associated endothelial cells. VEGF blockade can also "normalize" tumor vasculature, thereby leading to greater tumor oxygenation and drug penetration. This review will address completed and ongoing trials that have established and continue to clarify the effects of these agents in rectal cancer.
Over the past 30 years, the clinical management of rectal cancer has undergone significant evolution. Until the 1970s and 1980s, surgery was often the only therapeutic modality employed in the treatment of rectal cancer patients. However, patterns-of-failure analyses by Gunderson and others documented that local recurrence was a common and clinically significant pattern of failure, resulting in significant patient morbidity and death.[1,2] To reduce these high failure rates, sentinel trials from the Gastrointestinal Tumor Study Group (GITSG), North Central Cancer Treatment Group (NCCTG), and National Surgical Adjuvant Breast and Bowel Project (NSABP) evaluated different strategies of adjuvant radiation therapy and fluorouracil (5-FU)-based chemotherapy.[3-5] Study results demonstrated that adjuvant radiation therapy and chemotherapy improved local control and surgery vs surgery alone, leading to the routine integration of these modalities into daily practice in the United States.
Total Mesorectal Excision
More recently, British investigators and others have described innovations in surgical techniques for rectal cancer.[6,7] These reports indicate that a more complete dissection of the mesorectum (total mesorectal excision, or TME) leads to lower local failure rates. Results from single-institution studies show that local failure rates < 10% could be achieved with TME. These impressive results with TME raised questions about the need for adjuvant radiation therapy and stimulated a Dutch study randomizing 1,805 eligible patients with operable (including stage I) rectal cancer to preoperative radiation therapy followed by TME vs TME alone.[8] The results of this study demonstrated that patients receiving preoperative radiation therapy had improved local control vs patients undergoing TME only. Furthermore, the magnitude of improvement in local control with radiation therapy in this study may have been underestimated by the inclusion of stage I patients who have excellent outcomes with surgery only.
These findings have been supported by the preliminary results of a UK Medical Research Council (MRC) trial evaluating preoperative short-course radiation therapy vs selected postoperative combined-modality therapy.[9] In this phase III study, 1,350 patients with clinically resectable rectal cancer were randomized to short-course preoperative radiation therapy (25 Gy in 5 fractions) plus TME vs TME followed by selective postoperative chemoradiation (45 Gy in 25 fractions with 5-FU) for patients with tumor involvement of the circumferential resection margin. In addition, patients with stage III disease received postoperative chemotherapy.
For patients undergoing preoperative radiation therapy compared to selective postoperative chemoradiation, the local recurrence rates were significantly reduced (4.7% vs 11.1%). In addition, the investigators found a significant improvement in 3-year disease-free survival of patients undergoing preoperative radiation therapy vs selective postoperative chemoradiation (79.5% vs 74.9%). These results suggest that even with TME and adjuvant chemotherapy, preoperative radiation therapy improves outcomes over selective adjuvant postoperative chemoradiation for patients with high-risk disease.
Neoadjuvant Therapy
Because of the potential benefits of preoperative (vs postoperative) therapy, neoadjuvant trials have been pursued in rectal cancer patients. Two trials were initiated in the United States comparing these approaches. Both closed prematurely because of poor accrual. In contrast, German investigators successfully completed and published results of the CAO/ARO/AIO trial, comparing neoadjuvant chemoradiation to adjuvant chemoradiation.[10] This landmark study demonstrated that by simply altering the sequence of chemoradiotherapy to surgery, improved rates of compliance, local control, sphincter preservation, and acute/late toxicity could be achieved, validating the advantages of preoperative therapy. These findings have led to a new standard of care in the United States in the treatment of rectal cancer.
Recently, European trials have further evaluated the role of concurrent 5-FU–based chemotherapy with radiation therapy in the neoadjuvant treatment of rectal cancer. Trial results from the European Organisation for Research and Treatment of Cancer (EORTC), Fdration Francophone de la Cancrologie Digestive (FFCD), and Poland demonstrated improved pathologic response rates and local control with the addition of chemotherapy. However, these reports have not verified a survival advantage with the addition of concurrent 5-FU.[11-13] Improved disease-free and overall survival rates have been shown with the addition of newer chemotherapeutic agents (capecitabine [Xeloda], oxaliplatin [Eloxatin], irinotecan [Camptosar]) to conventional chemotherapies in patients with metastatic and locally advanced colorectal cancer.
These agents have now been incorporated into the testing of new strategies for neoadjuvant therapy of rectal cancer. Capecitabine is an oral fluoropyrimidine prodrug that is readily absorbed in the gastrointestinal tract and mimics the efficacy of continuous-infusion 5-FU while avoiding that strategy's associated risk of side effects and complications. Other options being evaluated for neoadjuvant therapy include the addition of oxaliplatin or irinotecan to 5-FU and radiation therapy. Early data from phase I/II trials suggest that an oxaliplatin dose of 60 mg/m2 can be combined safely with 5-FU–based chemotherapy and radiation therapy approaches with acceptable grade 3 toxicity.[14] In addition, promising rates of clinical and pathologic downstaging (25%–30%) have been reported. The NSABP R04 study is an ongoing phase III trial comparing preoperative radiation therapy and capecitabine with or without oxaliplatin with preoperative radiation therapy and continuous intravenous infusion of 5-FU with or without oxaliplatin in the treatment of patients with operable carcinoma of the rectum.
With increasing insight into the biochemical pathways within tumor cells that are related to tumor growth and spread, and the development of "targeted therapies" that block these pathways, much attention has turned to the use of these agents (in particular, cetuximab or gefitinib [Iressa] for EGFR and bevacizumab [Avastin] for VEGF) coupled with chemotherapy in the treatment of patients with advanced and metastatic rectal and colon cancer. For metastatic colorectal cancer patients, phase III trials have shown improved progression-free and overall survival rates with the use of these agents when combined with conventional chemotherapy. These agents are now being integrated in chemoradiotherapy protocols in phase I and II neoadjuvant studies of rectal cancer.
The goal of combining these agents with radiation therapy is to further enhance rates of tumor downstaging and sphincter preservation, local control, and survival. This review highlights the background, rationale, and results of combining cetuximab/gefitinib or bevacizumab with radiation therapy and chemotherapy in the treatment of localized rectal cancer.
EGFR Inhibitors
The epidermal growth factor receptor (EGFR, HER1, or ErbB1) is a 170-kD transmembrane glycoprotein with a cytoplasmic protein kinase domain. It is essential for tumor growth and division.[15] The receptor binds multiple ligands including epidermal growth factor and transforming growth factor–alpha. Dimerization or heterodimerization with other members of the EGFR family (HER2, HER3, HER4) of the extracellular domain then occurs with initiation of multiple downstream signaling pathways. These downstream signaling pathways again include the phosphorylation of MAPK through the ras/raf pathway.
Inhibition of these signaling pathways can result in cell growth arrest and apoptosis. EGFR inhibition has also been shown to decrease VEGF expression by cancer cells and tumor angiogenesis. EGFR overexpression is frequently detected in colorectal cancers and has been associated with poor prognosis.[16-18] Two types of EGFR inhibitors have been tested in patients with colorectal cancer: small-molecule EGFR tyrosine kinase inhibitors (gefitinib and erlotinib [Tarceva]) and monoclonal antibodies to EGFR (cetuximab and panitumumab [Vectibix]).
Gefitinib
Gefitinib is a selective inhibitor of the EGFR tyrosine kinase, resulting in interruption of mitogenic and antiapoptotic signals responsible for proliferation, growth, and angiogenesis. Preclinical studies in human colorectal and pancreatic cancer cell lines have shown enhanced cytotoxicity when gefitinib is combined with chemotherapy and radiation therapy.[19-22] The combination of capecitabine and gefitinib results in a synergistic effect relative to either agent alone in human cancer cell lines.[23]
Gefitinib with 5-FU–based chemotherapy appears to be feasible in patients with advanced colorectal cancer without a significant increase in severity of side effects.[24] In patients with metastatic colorectal cancer, a phase II trial including 115 patients treated with gefitinib as single-agent therapy showed no activity of gefitinib in this setting.[25] However, preliminary reports of small studies have described the combination of gefitinib with capecitabine as first-line therapy in patients with advanced colorectal cancer, with acceptable toxicity profiles and encouraging response rates.[26] Another preliminary report of gefitinib in combination with 5-FU, leucovorin, and oxaliplatin for the first line treatment of metastatic colorectal cancer showed a 74% response rate in a small cohort of 39 patients.[27] No survival data have been reported.
Erlotinib
Erlotinib, another inhibitor of EGFR tyrosine kinase, has also been tested in patients with metastatic colorectal cancer. A phase II study of single-agent erlotinib in patients with metastatic colorectal cancer who had not received more than one regimen of chemotherapy showed a time to disease progression of 56 days.[28] Another phase II study of capecitabine, oxaliplatin, and erlotinib in patients with previously treated metastatic colorectal cancer showed a 25% response rate with a median progression-free survival of 5.4 months.[29] Similar to studies of gefitinib for patients with metastatic colorectal cancer, studies of erlotinib have shown little or no activity of erlotinib as a single agent, and modest, if any, benefit in combination with chemotherapy.
Based on the potential radiosensitzing properties of capecitabine and gefitinib along with a potential synergistic effect of these agents when given in combination, investigators hypothesized that improved response rates, local control, and prevention of distant metastases may be achieved by combining these agents with radiation therapy. However, in a phase I trial from Duke University combining gefitinib, capecitabine, and radiation therapy in rectal cancer, the combination resulted in significant toxicity, and no recommended phase II dose could be determined.[30]
Cetuximab
Cetuximab is a chimeric anti-EGFR monoclonal antibody that binds to the extracellular domain of human EGFR. In a phase III trial, 329 patients with irinotecan-refractory metastatic colorectal cancer were randomized to cetuximab monotherapy and cetuximab plus irinotecan.[31] The rate of response, median time to progression, and median survival in the combination-therapy group was significantly higher than what was seen in the monotherapy group. Toxicity was more frequent in the combination group, but the severity and incidence were similar to what would be expected with irinotecan alone. Cetuximab has clinically significant activity when given alone or in combination with irinotecan in patients with irinotecan-refractory colorectal cancer. This agent is currently approved in the United States in combination with irinotecan for patients with metastatic colorectal cancer refractory to irinotecan or as a single agent for those who cannot tolerate irinotecan.
Overexpression of EGFR is regarded as a negative prognostic factor and is associated with resistance to radiation therapy. Therefore, EGFR has emerged as a promising target in radiation therapy. Cetuximab can be safely administered with conventional or hyperfractionated radiation therapy in patients with head and neck cancer. Moreover, cetuximab has been shown to improve survival in combination with curative-intent radiation therapy in patients with locally advanced head and neck cancer.[32]
In retrospective analyses, patients with EGFR-expressing rectal cancer undergoing neoadjuvant radiation therapy had a significantly lower disease-free survival and lower chance of achieving a pathologic complete response (pCR).[33-39] The positive data in metastatic colorectal cancer and synergy with radiation therapy in preclinical models provide a strong rationale for combining cetuximab with neoadjuvant radiation therapy and chemotherapy in rectal cancer. Phase I and I/II studies have been undertaken to evaluate this combination (Table 1).
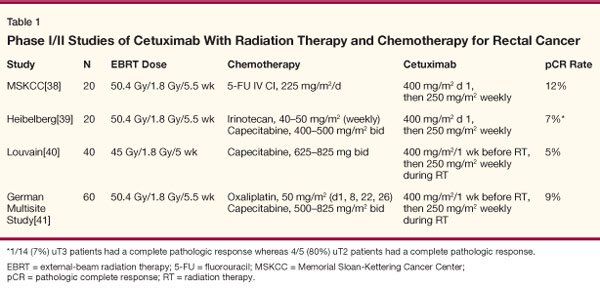
Investigators from Memorial Sloan-Kettering conducted a pilot trial to investigate the safety of cetuximab in combination with standard neoadjuvant protracted-infusion 5-FU and radiation therapy in patients with ultrasound T3/4 (uT3/4) or clinical T4 (cT4), or locally recurrent rectal adenocarcinoma.[40] Patients received cetuximab 400 mg/m2 on day 1 (250 mg/m2 weekly thereafter) and 5-FU (225 mg/m2 as a peripheral venous infusion) over 5.5 weeks with concurrent pelvic irradiation (50.4 Gy), and cetuximab (250 mg/m2 every week) for 4 additional weeks, followed by surgical resection 1 to 3 weeks later. Of the 20 patients enrolled, 18 completed radiation therapy and 17 underwent surgery. The pathologic complete response rate was 12%. These investigators concluded that cetuximab in combination with protracted-infusion 5-FU and concurrent radiation was feasible without synergistic or unexpected toxicities, and further investigation was warranted.
In a trial from Germany, 20 patients with endorectal ultrasound stage T2–4 or node-positive rectal cancer received a standard dose of cetuximab (400 mg/m2 on day 1 and 250 mg/m2 on days 8, 15, 22, and 29) and escalating doses of irinotecan and capecitabine with pelvic radiation therapy to a dose of 50.4 Gy.[41] Irinotecan at 40 mg/m2 and capecitabine at 500 mg/m2 bid were determined as the recommended doses for future studies. All 20 patients underwent surgery, and 1 of 14 patients with uT3/4 tumors had a complete pathologic response. Four of five patients with uT2 tumors had a pathologic complete response.
Investigators from Belgium reported on 40 patients with endoscopically staged T3/4 and/or N+ disease receiving preoperative radiation therapy (45 Gy) in combination with cetuximab (initial dose 400 mg/m2 given 1 week before the beginning of radiation followed by 250 mg/m2 weekly for 5 weeks) and capecitabine during the course of radiation therapy (first dose level: 650 mg/m2 orally twice daily; second dose level: 825 mg/m2 twice daily).[42] Four and six patients, respectively, were treated at the first and second dose levels of capecitabine. No dose-limiting toxicity occurred. Thirty additional patients were treated with capecitabine at 825 mg/m2 twice daily. The most frequent grade 1/2 side effects were acneiform rash (87%), diarrhea (65%), and fatigue (57%). Grade 3 diarrhea occurred in 15% of patients. Three grade 4 toxic effects were recorded: one myocardial infarction, one pulmonary embolism, and one pulmonary infection with sepsis. Two patients (5%) had a pathologic complete response.
In a phase I/II multicenter trial from Germany, 60 patients with clinical stage II–IV rectal cancer were treated with cetuximab and concurrent CAPOX (capecitabine plus oxaliplatin) and 50.4 Gy of pelvic irradiation.[43] Cetuximab was given in an initial dose of 400 mg/m2 7 days before the start of radiotherapy, then at 250 mg/m2 once weekly during radiotherapy (1.8–50.4 Gy). CAPOX was administered according to an established schedule of oxaliplatin (50 mg/m2 on days 1, 8, 22, 29) and capecitabine (days 1–14 and 22–35) at three dose levels-1,000, 1,300, 1,650 mg/m2-during the phase I portion of the study. The main endpoint of phase II was activity as assessed by the pCR rate.
A total of 13 patients were included in the phase I part, and the maximum tolerated dose was not reached.[43] Overall, 48 patients were treated at the recommended dose of capecitabine (1,650 mg/m2), and 45 patients underwent surgery. A pCR was observed in 4 patients (9%), whereas moderate, minimal, or no tumor regression was noted in a total of 53%. Grade 3/4 diarrhea was observed in 19% of patients. Postoperative complications of any grade occurred in 33% of patients. These investigators concluded that cetuximab can be safely combined with CAPOX/radiotherapy. The observed low rate of pCR should stimulate further preclinical investigations to establish the best sequence of triple combinations.
In summary, it appears that cetuximab can be safely combined with radiation therapy and chemotherapy in the neoadjuvant treatment of rectal cancer. Based on the results of current phase I or II studies, it is not clear that cetuximab results in enhanced tumor responses or downstaging with contemporary chemoradiation approaches.
Panitumumab
Panitumumab, a fully humanized monoclonal antibody that targets the EGFR, is also approved for treatment of metastatic colorectal cancer patients who have failed prior chemotherapy, including oxaliplatin- and/or irinotecan-containing regimens. Recently, a preliminary report of a trial of panitumumab in combination with bevacizumab and either FOLFOX (5-FU, leucovorin, and oxaliplatin) or FOLFIRI (5-FU, leucovorin, and irinotecan) therapy (Panitumumab Advanced Colorectal Cancer Evaluation, or PACCE) for patients with previously untreated metastatic colorectal cancer noted that the addition of panitumumab increased toxicity of therapy with a statistically significant decrease in overall survival for patients treated with panitumumab.
Antiangiogenic Agents
Increased levels of vascular endothelial growth factor (VEGF) expression have been found in the tumors and sera of patients with localized as well as metastatic colon and rectal cancer.[44-46] High VEGF expression has been associated with disease progression and inferior survival. In a study of 79 consecutive patients with node-positive rectal cancer treated by resection and adjuvant chemoradiation, those with tumors exhibiting VEGF overexpression were at statistically higher risk for the development of local recurrence and metastases.[47] Thus, inhibition of VEGF is a logical approach for the treatment of patients with rectal and colon cancer.
Metastatic Disease
A randomized phase II study (AVF0780g) first studied the efficacy and safety of the anti-VEGF antibody bevacizumab combined with 5-FU and leucovorin in patients with metastatic colon and rectal cancer.[48] Patients were randomized to bevacizumab (5 or 10 mg/kg) and 5-FU/leucovorin or 5-FU/leucovorin only. Patients receiving bevacizumab and 5-FU/leucovorin had statistically improved response rates and time to disease progression compared with patients receiving 5-FU/leucovorin only. No additional toxicity was observed with the addition of bevacizumab to 5-FU/leucovorin chemotherapy.
Based on these data, a phase III trial randomized 813 patients with previously untreated metastatic colorectal cancer to receive either IFL (irinotecan, 5-FU, and leucovorin) plus bevacizumab (402 patients) or IFL plus placebo (411 patients).[49] The median duration of survival was 20.3 months in the group given IFL plus bevacizumab, compared with 15.6 months in the group given IFL plus placebo (P < .001). The median duration of progression-free survival was 10.6 months in the group given IFL plus bevacizumab, compared with 6.2 months in the group given IFL plus placebo (P < .0001), and the corresponding rates of response were 44.8% and 34.8% (P = .004). The median duration of response was 10.4 months in the group given IFL plus bevacizumab, compared with 7.1 months in those given IFL plus placebo. Grade 3 hypertension was more common during treatment with IFL plus bevacizumab than with IFL plus placebo (11% vs 2.3%) but was easily managed. The investigators concluded that the addition of bevacizumab to 5-FU–based combination chemotherapy resulted in a statistically significant and clinically meaningful improvement in survival among patients with metastatic colorectal cancer.
In another trial, patients with advanced metastatic colon and rectal cancer who had been previously treated with 5-FU/irinotecan received either bevacizumab in combination with second-line therapy (FOLFOX4) or FOLFOX4 alone.[50] The addition of bevacizumab increased response rates (21.8% vs 9.2%, P = .0001), prolonged progression-free survival (7.2 vs 4.8 months, P < .0001), and improved overall survival (12.9 vs 10.8 months, P < .001). Side effects for this regimen included neuropathy, hypertension, bleeding, and bowel perforation. Whether combining bevacizumab with FOLFOX4 (or other chemotherapy regimens) will be the best option for first-line therapy in colorectal cancer is under investigation.
Finally, a Treatment Referral Center was established by the National Cancer Institute for patients with advanced metastatic colon and rectal cancer in the third-line setting (who had disease progression after irinotecan- and oxaliplatin-based chemotherapy), where no standard treatment options were available. Bevacizumab was added to a 5-FU/leucovorin regimen in these patients. While response data are not mature, bevacizumab does not seem to confer a survival advantage in this third-line setting.[51]
Local Disease
Although VEGF inhibition has been demonstrated to be beneficial for patients with metastatic colon and rectal cancer and enhanced their survival by several months, the magnitude of the effect of anti-VEGF therapy in patients with localized and nonmetastatic disease is not known. This issue is under investigation in several phase I/II clinical trials that combine bevacizumab with cytotoxic regimens for localized rectal cancer.
Recent experimental studies in human tumor xenografts as models of primary tumors have demonstrated that VEGF blockade serves as a potent and nontoxic enhancer of radiation therapy, reducing tumor interstitial pressure (a known barrier to drug delivery to tumors), and in some cases, reducing tumor hypoxia (a known barrier to radiation therapy).[52-55] The vascular normalization paradigm as proposed by Jain and colleagues in 2001 may explain these observations. In this paradigm, antiangiogenic agents transiently normalize abnormal tumor vasculature (formed as a result of excessive local production of angiogenic factors).[56,57] With structural and functional remodeling of the tumor blood vessels, concentration of oxygen and penetration of macromolecules is improved and tumor response to chemotherapy and radiation therapy is enhanced.
Chemoradiation Combinations
• Bevacizumab/5-FU-To test the hypothesis that inhibition of VEGF is safe and results in clinical benefit and enhancement of radiation therapy response, a neoadjuvant phase I/II trial with bevacizumab in combination with 5-FU and radiation therapy in patients with T3 or T4 rectal cancer was undertaken.[58,59] Bevacizumab was delivered as a 90-minute infusion on day 1 of each cycle (Figure 1). The dose was escalated in successive cohorts of six patients, beginning at 5 mg/kg and followed by 10 mg/kg. Infusional 5-FU was administered over 24 hours each day at a fixed dose of 225 mg/m2 throughout each treatment week of cycles 2 through 4. External-beam irradiation was also administered during cycles 2 to 4 for a total dose of 50.4 Gy in 28 fractions over 5.5 weeks.
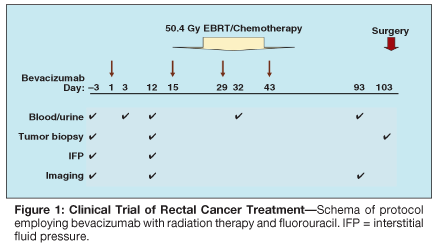
The primary clinical objective of this study was to determine the maximum tolerated dose of bevacizumab when delivered concurrently with 5-FU and external-beam radiation therapy in patients with T3 or T4 rectal cancer prior to surgery. A major parallel goal of this study was to clarify through correlative studies the mechanisms by which bevacizumab inhibits angiogenesis and improves the outcome of other therapeutic modalities in the treatment of this malignancy.
The phase I portion of the study has been completed.[58,59] The first six patients treated with the combination of bevacizumab at the 5-mg/kg dose level with chemotherapy and radiation therapy tolerated this treatment without difficulty. All six patients underwent surgery without complication. In contrast, two of five patients in the second cohort who were given "high-dose" bevacizumab (10 mg/kg) with chemotherapy and radiation therapy experienced grade 3/4 dose-limiting diarrhea and colitis during the combined treatment. Following recovery from toxicity, these patients were able to resume and complete radiation therapy and 5-FU treatment. Because of these dose-limiting toxicities, only five patients were enrolled at the 10-mg/kg dose. All the patients underwent surgery. Of note, one patient on high-dose bevacizumab experienced a pulmonary embolus on day 1 postoperatively and recovered completely with anticoagulation. Another patient in this cohort developed ileostomy obstruction with stent-related ileal perforation 10 days after resection, requiring laparotomy and ileostomy revision.
The design of this trial permitted a unique opportunity to evaluate the effect of bevacizumab alone (cycle 1) on rectal cancer prior to its concurrent administration (cycles 2–4) with radiation therapy and chemotherapy. Correlative studies were undertaken to clarify the mechanism of action of bevacizumab on rectal cancer. At 12 days after the first bevacizumab infusion, patients underwent repeat flexible sigmoidoscopy with tumor biopsy, tumor interstitial pressure measurement, perfusion computed tomography (CT) scan to measure blood flow, positron-emission tomography 18F-fluorodeoxyglucose (PET-FDG) scan, and analysis of blood and urine for angiogenesis markers.
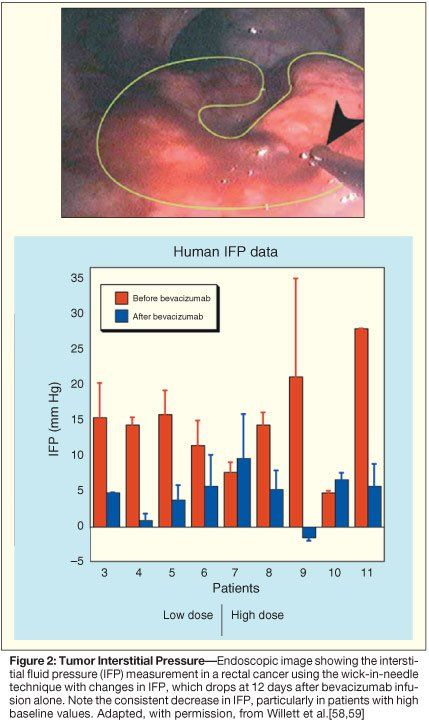
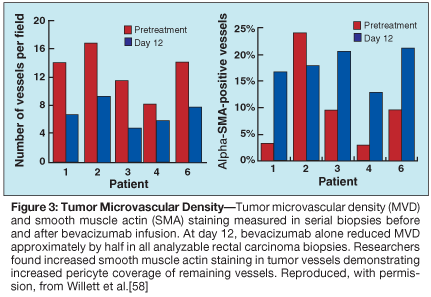
At day 12, 1 of 11 patients exhibited a partial clinical response, and a number of antivascular effects induced by bevacizumab were observed. Tumor interstitial pressure measurements were statistically lower following bevacizumab administration (Figure 2). These data are consistent with preclinical data and support the normalization hypothesis. Tumor vascular density measurements by immunohistochemistry (Figure 3) and blood flow parameters by perfusion CT (not shown) also dropped following bevacizumab infusion.[58,59] In contrast, FDG uptake in the tumors measured on PET scans remained constant (Figure 4). Despite these decreases in tumor vascular density and blood flow, tumor metabolism as assessed by FDG activity was unchanged, thus supporting the normalization hypothesis. Further support of the normalization hypothesis has been the observation of increased pericyte coverage in tumor vessels following bevacizumab administration (Figure 3).
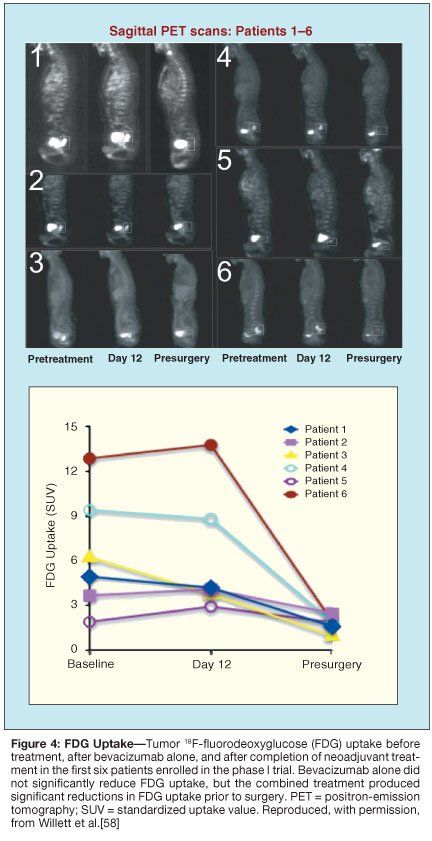
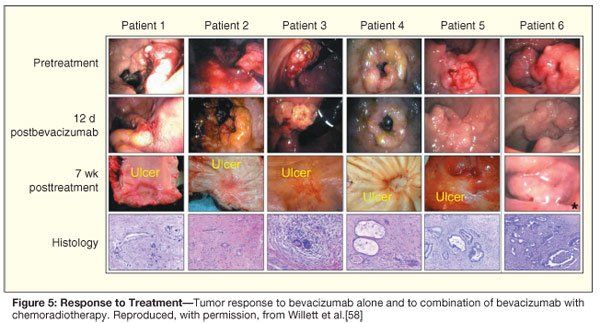
The findings of this completed phase I study of bevacizumab with chemoradiation in rectal cancer are consistent with an antivascular and vascular normalizing effect of VEGF blockade on the tumor. Tumor responses evaluated by endoscopy, CT, and PET at 7 weeks after completion of the combined treatment are encouraging with evidence of tumor regression and decreased FDG activity. At surgery, pathologic examination of the tissue usually revealed minimal microscopic disease (Figure 5). Complete pathologic responses were seen with this combined regimen at both 5- and 10-mg/kg dosing of bevacizumab, even in the case of a large, invasive, ultrasound stage T4 carcinoma.[60] The efficacy signals seen with these regimens remain to be confirmed in the ongoing phase II studies, and established in future randomized trials of antiangiogenics with chemoradiation.
• Capecitabine/Oxaliplatin/Bevacizumab-A phase I trial from Duke University evaluated the combination of concurrent capecitabine, oxaliplatin, and bevacizumab in patients with stage II–IV rectal cancer. Patients were treated with escalating doses of capecitabine and oxaliplatin, and a fixed dose of bevacizumab (15 mg/kg on day 1 and 10 mg/kg on days 8 and 22). At dose level 1, patients were initiated on oxaliplatin at 50 mg/m2 weekly and capecitabine at 625 mg/m2 bid. External-beam irradiation was administered to a total dose of 50.4 Gy in 28 fractions over 5.5 weeks.
At dose level 1, all patients initially experienced good tolerance of treatment without dose-limiting toxicity. Two patients enrolled at dose level 2 had dose-limiting toxicities of diarrhea and tenesmus-type symptoms. Additional patients were then enrolled at dose level 1, resulting in one dose-limiting toxicity (diarrhea). Therefore, the recommended phase II doses were bevacizumab, 15 mg/kg on day 1, 10 mg/kg on days 8 and 22; oxaliplatin, 50 mg/m2 weekly; and capecitabine, 625 mg/m2 bid during radiation days.[61] Two patients had a pathologic complete response, and three patients had microscopic disease only.
These and other data have stimulated further investigation of the combination of anti-VEGF therapy in conjunction with conventional radiation and chemotherapy in patients with rectal cancer (Table 2).

Summary
The positive data in metastatic colorectal cancer and synergy with radiation therapy seen in preclinical models provide a strong rationale for combining cetuximab with neoadjuvant radiation therapy and chemotherapy in rectal cancer. Phase I and II studies evaluating this approach have recently been completed. It appears that cetuximab can be safely combined with radiation therapy and chemotherapy in the neoadjuvant treatment of rectal cancer. However, it is not clear that cetuximab results in enhanced tumor responses or downstaging with contemporary chemoradiation approaches.
Bevacizumab may lead to indirect killing of cancer cells by damaging tumor blood vessels, and may increase the radiosensitivity of tumor-associated endothelial cells. VEGF blockade can also "normalize" tumor vasculature, thereby leading to greater tumor oxygenation (a known radiosensitizer) and drug penetration. Phase I/II trials have demonstrated that bevacizumab can be safely combined with neoadjuvant radiation therapy and chemotherapy in rectal cancer patients. In addition, bevacizumab has antivascular effects that support the normalization hypothesis in this clinical setting. Ongoing phase II studies will further clarify the mechanisms of action and efficacy of this agent in locally advanced rectal cancer.
References:
1. Gunderson LL, Sosin H: Areas of failure found at reoperation (second or symptomatic look) following "curative surgery" for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer 34:1278-1292, 1974.
2. Rich T, Gunderson LL, Lew R, et al: Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer 52:1317-1329, 1983.
3. Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med 312:1465-1472, 1985.
4. Krook JE, Moertel CG, Gunderson LL, et al: Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 324:709-715, 1991.
5. Wolmark N, Wieand HS, Hyams DM, et al: Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 92:388-396, 2000.
6. Heald RJ: The 'Holy Plane' of rectal surgery. J R Soc Med 81:503-508, 1988.
7. Heald RJ, Moran BJ, Ryall RD, et al: Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 133:894-899, 1998.
8. Kapiteijn E, Marijnen CA, Nagtegaal ID, et al: Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638-646, 2001.
9. Sebag-Montefiore D, Steele R, Quirke P, et al: Routine short course pre-op radiotherapy or selective post-op chemoradiotherapy for resectable rectal cancer: Preliminary results of the MRCX CR07 randomized trial (abstract 3511). J Clin Oncol 24(18S):148s, 2006.
10. Sauer R, Becker H, Hohenberger W, et al: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731-1740, 2004.
11. Bosset JF, Collette L, Calais G, et al: Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114-1123, 2006.
12. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al: Sphincter preservation following preoperative radiotherapy for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 72:15-24, 2004.
13. Gerard JP, Conroy T, Bonnetain F, et al: Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 24:4620-4625, 2006.
14. Rodel C, Liersch T, Hermann RM, et al: Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 25:110-117, 2007.
15. Saeki T, Salomon DS, Johnson GR, et al: Association of epidermal growth factor-related peptides and type I receptor tyrosine kinase receptors with prognosis of human colorectal carcinomas. Jpn J Clin Oncol 25:240-249, 1995.
16. Khorana A, Ryan C, Eberly S, et al: EGFR expression and survival in stage II, III and IV colon cancer (abstract 1272). Proc Am Soc Clin Oncol 22:317, 2003.
17. Nicholson RI, Gee JM, Harper ME: EGFR and cancer prognosis. Eur J Cancer 37(suppl 4):S9-15, 2001.
18. Raymond E, Faivre S, Armand JP: Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs 60(suppl 1):15-23; discussion 41-2, 2000.
19. Ciardiello F, Caputo R, Bianco R, et al: Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res 6:2053-2063, 2000.
20. Raben D, Helfrich BA, Chan D, et al: ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, alone and in combination with radiation and chemotherapy as a new therapeutic strategy in non-small cell lung cancer. Semin Oncol 29:37-46, 2002.
21. Raben D, Phistery M, Helfrich B, et al: ZD1839, a selective epidermal growth factor receptor trosine kinase inhibitor enhances radiation-induced cytotoxicity in human pancreatic and cholangiocarcinoma cell lines in vitro. Presented at Gastrointestinal Cancer Research Conference; Orlando, Fla; November 2000.
22. Williams KJ, Telfer BA, Stratford IJ, et al: ZD1839 ('Iressa'), a specific oral epidermal growth factor receptor-tyrosine kinase inhibitor, potentiates radiotherapy in a human colorectal cancer xenograft model. Br J Cancer 86:1157-1161, 2002.
23. Magne N, Fischel JL, Dubreuil A, et al: ZD1839 (Iressa) modifies the activity of key enzymes linked to fluoropyrimidine activity: Rational basis for a new combination therapy with capecitabine. Clin Cancer Res 9:4735-4742, 2003.
24. Fisher G, Kuo T, Cho C, et al: A phase II study of gefitnib in combination with FOLFOX-4 (IFOX) in patients with metastatic colorectal cancer (abstract 3514). Proc Am Soc Clin Oncol 23:249, 2004.
25. Rothenberg ML, LaFleur B, Levy DE, et al: Randomized phase II trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. J Clin Oncol 23:9265-9274, 2005.
26. Zeuli M, Gelibter A, Nardoni C, et al: A feasibility study of gefitinib in association with capecitabine (CAP) and oxaliplatin (OXA) as first-line treatment in patients with advanced colorectal cancer (abstract 3748). Proc Am Soc Clin Oncol 22:306, 2004.
27. Zampino MG, Lorizzo K, Massacesi C, et al: First-line gefitinib combined with simplified FOLFOX-6 in patients with epidermal growth factor receptor-positive advanced colorectal cancer (abstract 3659). Proc Am Soc Clin Oncol 23(16S): 285s, 2005.
28. Townsley CA, Major P, Siu LL, et al: Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer 94:1136-1143, 2006.
29. Meyerhardt JA, Zhu AX, Enzinger PC, et al: Phase II study of capecitabine, oxaliplatin, and erlotinib in previously treated patients with metastastic colorectal cancer. J Clin Oncol 24:1892-1897, 2006.
30. Czito BG, Willett CG, Bendell JC, et al: Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: Phase I trial results. J Clin Oncol 24:656-662, 2006.
31. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
32. Bonner JA, Harari PM, Giralt J, et al: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567-578, 2006.
33. Azria D, Bibeau F, Barbier N, et al: Prognostic impact of epidermal growth factor receptor (EGFR) expression on loco-regional recurrence after preoperative radiotherapy in rectal cancer. BMC Cancer 5:62, 2005.
34. Giralt J, de las Heras M, Cerezo L, et al: The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: A multicenter, retrospective analysis. Radiother Oncol 74:101-108, 2005.
35. Giralt J, Eraso A, Armengol M, et al: Epidermal growth factor receptor is a predictor of tumor response in locally advanced rectal cancer patients treated with preoperative radiotherapy. Int J Radiat Oncol Biol Phys 54:1460-1465, 2002.
36. Kim JS, Kim JM, Li S, et al: Epidermal growth factor receptor as a predictor of tumor downstaging in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 66:195-200, 2006.
37. Li S, Kim JS, Kim JM, et al: Epidermal growth factor receptor as a prognostic factor in locally advanced rectal-cancer patients treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 65:705-712, 2006.
38. Spindler KL, Nielsen JN, Lindebjerg J, et al: Prediction of response to chemoradiation in rectal cancer by a gene polymorphism in the epidermal growth factor receptor promoter region. Int J Radiat Oncol Biol Phys 66:500-504, 2006.
39. Zhang W, Park DJ, Lu B, et al: Epidermal growth factor receptor gene polymorphisms predict pelvic recurrence in patients with rectal cancer treated with chemoradiation. Clin Cancer Res 11:600-605, 2005.
40. Chung K, Minsky B, Schrag D, et al: Phase I trial of preoperative cetuximab with concurrent continuous infusion 5-fluorouracil and pelvic radiation in patients with local-regionally advanced rectal cancer (abstract 3560). J Clin Oncol 24(18S):161s, 2006.
41. Hofheinz RD, Horisberger K, Woernle C, et al: Phase I trial of cetuximab in combination with capecitabine, weekly irinotecan, and radiotherapy as neoadjuvant therapy for rectal cancer. Int J Radiat Oncol Biol Phys 66:1384-1390, 2006.
42. Machiels JP, Sempoux C, Scalliet P, et al: Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann Oncol 18:738-744, 2007.
43. Rodel C, Hipp M, Liersch T, et al: Cetuximab, capecitabine, oxaliplatin, and radiation therapy as preoperative treatment in rectal cancer. Presented at the 48th Annual Meeting of the American Society for Therapeutic Radiology and Oncology. Philadelphia, November 5-9, 2006.
44. Chin KF, Greenman J, Gardiner E, et al: Pre-operative serum vascular endothelial growth factor can select patients for adjuvant treatment after curative resection in colorectal cancer. Br J Cancer 83:1425-1431, 2000.
45. Hyodo I, Doi T, Endo H, et al: Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer 34:2041-2045, 1998.
46. Nanashima A, Ito M, Sekine I, et al: Significance of angiogenic factors in liver metastatic tumors originating from colorectal cancers. Dig Dis Sci 43:2634-2640, 1998.
47. Cascinu S, Graziano F, Catalano V, et al: Vascular endothelial growth factor (VEGF), p53, and BAX expression in node positive rectal cancer (abstract 595). Proc Am Soc Clin Oncol 20:150a, 2001.
48. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al: Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60-65, 2003.
49. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
50. Giantonio BJ, Levy DE, O'Dwyer PJ, et al: A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol 17:1399-1403, 2006.
51. National Cancer Institute: Bevacizumab (Avastinâ„¢) for metastatic colorectal cancer. Available at www.cancer.gov/newscenter/pressreleases/bevacizumab. Accessed June 18, 2007.
52. Kozin SV, Boucher Y, Hicklin DJ, et al: Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res 61:39-44, 2001.
53. Lee CG, Heijn M, di Tomaso E, et al: Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 60:5565-5570, 2000.
54. Tong RT, Boucher Y, Kozin SV, et al: Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64:3731-3736, 2004.
55. Winkler F, Kozin SV, Tong RT, et al: Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553-563, 2004.
56. Jain RK: Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med 7:987-989, 2001.
57. Jain RK: Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 307:58-62, 2005.
58. Willett CG, Boucher Y, di Tomaso E, et al: Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145-147, 2004.
59. Willett CG, Boucher Y, Duda DG, et al: Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 23:8136-8139, 2005.
60. Willett CG, Duda DG, di Tomaso E, et al: Complete pathological response to bevacizamab in advanced rectal cancer. Nat Clin Pract Oncol 4:316-321, 2007.
61. Czito BG, Bendell JC, Willett CG, et al: Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int J Radiat Oncol Biol Phys 68:472-478, 2007.