Thrombopoietin: Biology and Potential Clinical Applications
After an almost 40-year search for a primary regulatory of platelet production, thrombopoietin has recently been purified and cloned. Thrombopoietin regulates all stages in the production of platelets by promoting both the
ABSTRACT: After an almost 40-year search for a primary regulatory of platelet production, thrombopoietin has recently been purified and cloned. Thrombopoietin regulates all stages in the production of platelets by promoting both the proliferation of megakaryocyte progenitors and their maturation into platelet-producing megakaryocytes. In preclinical studies in normal mice and nonhuman primates, administration of thrombopoietin resulted in a rapid rise in platelet counts to levels previously unattainable with other thrombopoietic cytokines. In myelosuppressed animal models, use of thrombopoietin following chemotherapy, radiation, or stem-cell transplantation accelerated megakaryocyte and platelet recovery. Thrombopoietin has rapidly moved from the laboratory to the clinic in the last 3 years. Preliminary results of clinical trials using truncated or full-length forms of the molecule indicate that thrombopoietin is a powerful stimulus to the production of megakaryocytes and normal platelets in humans and enhances platelet recovery following chemotherapy. Although the peripheral effect is selective on platelet lineage, thrombopoietin mediates stimulatory effects on progenitors of multiple cell lineages at the bone marrow level and mobilizes progenitor cells into the peripheral blood. These biological effects suggest that thrombopoietin holds promise as a useful agent for the prevention and treatment of thrombocytopenia in cancer patients and for other disorders of thrombocytopenia. [ONCOLOGY 12(11):1597-1608, 1998]
Introduction
Thrombocytopenia is a well-recognized problem that occurs in patients with malignancies. Although multiple etiologic factors of primary (eg, intrinsic bone marrow disease) or secondary (eg, tumor-induced coagulopathy, immune-mediated thrombocytopenia, hypersplenism-related platelet sequestration) origin can contribute to thrombocytopenia in cancer patients, the most common etiology of clinically significant thrombocytopenia is the increasing use of myelosuppressive chemotherapy.
Administration of antibiotics, transfusion of blood products, and modifications in chemotherapy doses have been the major means of combating chemotherapy-induced hematologic toxicity. Over the past decade, the clinical availability of hematopoietic growth factors, such as granulocyte colony-stimulating factor (G-CSF, filgrastim [Neupogen]), granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim [Leukine]), and erythropoietin (Epogen, Procrit) have helped reduce clinical complications associated with granulocyte and erythroid cell lineages. Thrombocytopenia has been managed primarily by platelet transfusions and chemotherapy dose reductions.
TABLE 1

Chemotherapeutic Regimens Associated With Significant Thrombocytopenia
Severe thrombocytopenia is a frequent clinical problem in the management of leukemia and pediatric malignancies, as well as in the setting of bone marrow or peripheral blood progenitor-cell transplantation. Although clinically significant thrombocytopenia does not commonly occur with most standard chemotherapeutic regimens used in the treatment of solid tumors, cumulative thrombocytopenia can be problematic with multiple cycles of certain chemotherapeutic regimens used in the treatment of lymphoma, sarcoma, breast cancer, ovarian cancer, and germ cell tumors (Table 1).[1-11] Furthermore, the concept that the dose intensity of chemotherapy may be important in improving overall outcome has led to the use of more aggressive, potentially curative regimens in patients with chemosensitive malignancies.
The use of peripheral blood progenitor cells has significantly reduced the duration of thrombocytopenia in patients receiving myeloablative or highly myelosuppressive therapy. However, 80% of patients undergoing autologous or allogeneic marrow transplantation continue to require transfusions 3 weeks after transplantation, as compared with 20% of patients receiving autologous peripheral blood progenitor cell transplants.[12]
The use of platelet transfusions to manage severe thrombocytopenia has increased approproximately twofold in the United States from 1982 to 1992.[13,14] Advances in organ transplantation and cardiac surgery and the use of aggressive chemotherapy in patients with treatment- responsive malignancies have all contributed to this marked increase in the need for platelets and to rising health-care costs.
The total national direct medical cost of treating chemotherapy-induced thrombocytopenia was estimated to range from $146 to $233 million in 1993.[15] In a recently conducted, observational study of platelet utilization in patients undergoing myeloablative treatment and stem-cell transplantation at 18 transplant centers in the United States and Canada, which used conservative cost figures for platelet transfusions, the average 60-day platelet cost per patient was estimated at $3,500 for autologous peripheral blood progenitor-cell support and $9,200 for allogeneic bone marrow transplantation (BMT).[12]
Although platelet transfusions may decrease the risk of fatal bleeding complications, repeated use of platelets increases the risks of transmission of infectious diseases, transfusion reactions, alloimmunization, and graft-vs-host disease.[14] All of these factors have motivated the search for an agent that could stimulate platelet production and prevent or ameliorate thrombocytopenia.
Megakaryocytopoiesis and Platelet Production
Platelets are small, anucleated cells that play an essential role in maintaining normal hemostasis. The normal circulating platelet count (range, 150 to 450 × 103/L) varies greatly between individuals but remains fairly constant in any one individual; there is minimum day-to-day variation in platelet count throughout life unless altered by a disease process or a change in physiologic condition.[16]
The body maintains a constant circulating mass of platelets through the process of megakaryocytopoiesis. Peripheral blood platelets are derived from megakaryocytes, which are located primarily in the bone marrow.[17] However, their large size and ability to attain a multilobular nucleus with a polyploid complement of DNA and abundant cytoplasm allow megakaryocytes to produce several thousand platelets per cell.
During megakaryocytopoiesis, pluripotent hematopoietic cells undergo commitment to the megakaryocyte lineage. The burst-forming unit-megakaryocyte (BFU-MK) is the most primitive megakaryocyte progenitor cell.[18] The BFU-MKs take 21 days to develop in culture, and their immunophenotypic cell surface markers are CD34+, c-kit+ and HLA-DR-. The BFU-MKs differentiate and produce colony-forming unit-megakaryocytes (CFU-MKs), which take 12 days to appear in culture and carry cell surface markers CD34+, c-kit+, and HLA-DR+.
Colony-forming unit-megakaryocytes give rise to megakaryocytes (MKs), which lose their capacity to undergo mitosis but retain their capacity for endoreduplication. Megakaryocytes become large polyploid cells (up to 128N) with maturation of cytoplasm and acquisition of biochemical properties characteristic of functional platelets. Platelets are produced by transendothelial cytoplasmic fragmentation and have a life span of approximately 10 days.[17]
Development of Thrombopoietin
The concept of a lineage-specific regulator involved in platelet development originated nearly 40 years ago. The term "thrombopoietin" was first used in 1958 to describe a humoral regulator present in thrombocytopenic plasma that restored platelet counts to a normal level after the onset of the thrombocytopenic state.[19] Since then, many groups have attempted to isolate and purify thrombopoietin from thrombocytopenic plasma, serum, and other physiologic sources.
A major step in the search for thrombopoietin came in 1992 with the cloning of the cellular homolog of the viral oncogene, c-Mpl.[20] This line of research began in 1986 with the identification of the murine retrovirus myeloproliferative leukemia virus (MPLV).[21] The responsible viral oncogene, v-Mpl, was cloned in 1990[22] and the corresponding cellular proto-oncogene, c-Mpl, was identified in 1992.[20] Sequence analysis of c-Mpl suggested homology to the genes encoding a family of hematopoietic growth factor receptors.[20]
Subsequent studies established the link between c-Mpl and megakaryocytopoiesis.[23] The expression of c-Mpl appeared to be restricted to CD34+ hematopoietic stem cells, megakaryocytes, and platelets. Furthermore, c-Mpl antisense oligonucleotides selectively inhibited megakaryocytic colony form- ation in vitro without affecting the growth of erythroid or granulocyte-macrophage colonies,[23] indicating that a putative ligand for c-Mpl may be a lineage-specific regulator of megakaryocytopoiesis.
From that point on, the search for the c-Mpl ligand was aggressively pursued. In 1994, five different groups [24-28] using three distinct strategies reported on the identification, purification, and cloning of cDNA for the Mpl ligand. The observation that all of the megakaryocyte colony- and platelet-stimulating activity from thrombocytopenic plasma can be removed by recombinant c-Mpl further confirmed that this ligand is, indeed, thrombopoietin[29]; the ligand was also named megakaryocyte growth and development factor (MGDF)[24] and megapoietin[26] by different investigators.
Structure and Properties of Thrombopoietin
FIGURE 1

Structure of Human Thrombopoietin
The human thrombopoietin gene is located on chromosome 3q27 and consists of seven exons joined to six introns.[28,30] The precursor protein product is made of 353 amino acids, and cleavage of a signal peptide at the amino terminus produces a mature peptide of 332 amino acids with two domains (Figure 1).
The amino terminal domain consists of 153 amino acids, including 4 cysteine residues, and has 23% homology with erythropoietin.[25] The similarity increases to 50% when conservative amino acids are taken into account.
The carboxy terminal domain consists of 179 amino acids and has several N-linked glycosylation sites.[17,31] Whereas the amino terminal domain is sufficient for the thrombopoietic effects of the molecule, the carboxy terminal domain is likely important in maintaining its circulating half-life.[31]
The major site of thrombopoietin production is the liver (fetal and adult).[25,27,30] Other sites expressing thrombopoietin mRNA include bone marrow, kidney, brain, testes, and spleen.[17,25,27,30]
The c-Mpl receptor, the receptor for thrombopoietin, is a transmembrane protein with an N-terminal extracellular domain and a C-terminal intracellular domain. The receptor expression is largely restricted to the tissues that support hematopoiesis, ie, bone marrow cells, spleen, and fetal liver, and is mostly expressed on CD34+ cells, cells of megakaryocyte lineage, and platelets.[20,23,32] The gene for the c-Mpl receptor is located on mouse chromosome 4[20] and human chromosome 1p34.[33]
The c-Mpl receptor binds to the ligand (thrombopoietin), which results in proliferation and differentiation of megakaryocyte progenitors.[29] Activation of the c-Mpl receptor results in the activation of the JAK/STAT pathway and possibly the Ras signal transduction pathway.[34] With receptor activation, there is an increase in megakaryocyte number, increased nuclear mass, increased ploidy, and cytoplasmic maturation and release of platelets.[31]
In Vitro Properties
FIGURE 2
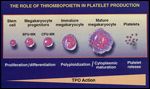
Regulation of Megakaryocytopoiesis by Thrombopoietin
In vitro studies have demonstrated that thrombopoietin stimulates all stages of megakaryocytopoiesis.[26,31,35,36] It acts as a megakaryocyte colony-stimulating factor in the early stages of megakaryocytopoiesis to stimulate the proliferation of megakaryocytic progenitors and in later stages to promote maturation of megakaryocytes into large polyploid cells capable of producing platelets (Figure 2). In suspension cultures, thrombopoietin increases the size and ploidy of megakaryocytes and the expression of Ib and IIb/IIIa glycoproteins on the cell surface.[18,37,38] Thrombopoietin-induced megakaryocytes express increased formation of demarcation membranes, platelet-specific granules, and platelet territories.[39,40]
Studies have demonstrated that thrombopoietin is essential for full megakaryocyte maturation in cell cultures.[37] In these studies, the soluble form of the Mpl receptor was used to neutralize all of thrombopoietin’s biological activity. The addition of this soluble receptor terminated megakaryocyte formation despite the presence of C-kit ligand (stem cell factor), interleukin-6 (IL-6), or inter-leukin-11 (IL-11). However, in the presence of interleukin-3 (IL-3), production of megakaryocytes decreased but did not cease entirely; in addition, megakaryocytes lacked demarcation membranes and platelet-specific granules and had low ploidy (4N) levels.
The effects of thrombopoietin are not limited to megakaryocytopoiesis. In vitro studies demonstrate that, in the presence of erythropoietin, thrombopoietin enhances the growth of erythroid progenitor cells.[41,42] In addition, thrombopoietin directly stimulates primitive hematopoietic stem cells in the presence of early-acting cytokines (IL-3 or C-kit ligand) and exhibits both proliferative and differentiative effects on these progenitor cells.[42-44]
Physiologic Role and Regulation
The role of thrombopoietin as a physiologic regulator of platelet production has been supported by the inverse relationship between the platelet count and serum thrombopoietin level in animal models of thrombocytopenia induced by antiplatelet-antiserum or by chemotherapy or radiation treatment, as well as in cancer patients following myeloablative chemotherapy and bone-marrow transplantation.[45-49]
The importance of the c-Mpl ligand and receptor system in physiologic regulation of platelet production in vivo is further demonstrated by the generation of c-Mpl and thrombopoietin knock-out mice. Mice genetically altered to be defective in c-Mpl or the thrombopoietin gene exhibit an 85% reduction in peripheral platelet count, as well as decreased bone marrow and splenic megakaryocytes.[50,51]
In addition, the lack of thrombopoietin or c-Mpl results in significant reductions in the levels of erythroid, myeloid, and multipotential progenitor cells. This indicates that thrombopoietin acts not only on cells committed to megakaryocyte lineage but also on primitive hematopoietic progenitor cells.[52,53]
As in other thrombocytopenic animal models, circulating thrombopoietin levels are elevated in c-mpl-/- mice.[50,54] However, there is no detectable difference between the c-mpl-/-and c-mpl+/+ mice with respect to thrombopoietin mRNA levels in any tissue. This suggests that transcriptional regulation of the thrombopoietin gene is not involved in the increased level of thrombopoietin observed during thrombocytopenia.[54]
An alternative theory of thrombopoietin regulation states that circulating thrombopoietin levels are regulated by platelet mass.[55] Recent studies support this hypothesis.[16,17,55] Injection of normal platelets into c-mpl-/- mice resulted in a decrease in plasma thrombopoietin level and normalization of platelet levels.[54,55] These findings suggest that thrombopoietin is constitutively synthesized and released into the circulation by the liver and/or kidney. Under normal circumstances, platelets clear most of the thrombopoietin from the circulation via binding to the c-Mpl receptors.[51,54,55]
In disorders of platelet production, such as aplastic anemia,[56] or in patients undergoing chemotherapy or radiotherapy, plasma thrombopoietin levels are elevated. This is due most likely to a decrease in the clearance mechanism related to the low platelet mass. However, in conditions in which low platelet numbers are accompanied by normal or increased numbers of megakaryocytes (such as idiopathic thrombocytopenic purpura), circulating thrombopoietin levels remain normal since thrombopoietin binds to megakaryocytes via c-Mpl receptors.[56]
Preclinical Biology
In normal mice, administration of thrombopoietin causes a rapid, four- to sixfold rise in circulating platelet count, along with increases in number, size, and ploidy of bone marrow and splenic megakaryocytes.[27,35,36] The magnitude of platelet response to thrombopoietin is higher than has previously been achieved with other thrombopoietic cytokines. In addition, thrombopoietin also increases the number of burst-forming unit-erythroid (BFU-E) in the bone marrow and spleen.[57]
Although, in normal animals, thrombopoietin exerts its effects exclusively on platelets, in the myelosuppressed murine model, administration of recombinant murine thrombopoietin accelerated not only platelet recovery[58] but also red blood cell recovery.[57,59,60] Thrombopoietin-treated animals showed an increase in megakaryocytic as well as myeloid and erythroid progenitors in the bone marrow and spleen. Thus, the effect of thrombopoietin on multiple cell lineages at the progenitor-cell level, along with increased levels of other endogenous cytokines in a severely myelosuppressed model, may enhance recovery of other cell lineages.
In the myelosuppressed nonhuman primate model, thrombopoietin also ameliorated platelet nadirs following whole-body irradiation.[61] These preclinical trials have shown major activity of this cytokine without significant toxicity.
Clinical Development of Thrombopoietin
Since its cloning, thrombopoietin has moved from the laboratory to the clinic in the last 3 years. Two forms of recombinant human thrombopoietin have been developed for human clinical investigation. A truncated version of thrombopoietin, developed by Amgen, consists of an N-terminal domain, which has an amino acid sequence identical to the N-terminal 163 amino acids of the native c-Mpl ligand and has a half-life of 1.5 hours. This molecule is covalently bound to polyethylene glycol to increase its half-life, and is referred to as pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF).[62]
A full-length glycosylated molecule produced in a genetically modified mammalian cell line has been developed by Genentech, Inc., and is referred to as recombinant human thrombopoietin (rhTPO). Both of these molecules have been evaluated in phase I clinical trials, and the initial results of these trials have been reported.
Clinical Trials of PEG-rHuMGDF
A phase I, randomized, placebo-controlled clinical trial evaluated PEG-rHuMGDF in cancer patients both before and after they received chemotherapy.[63,64] In the prechemotherapy phase of the trial, 15 patients were treated with either placebo (N = 4) or PEG-rHuMGDF (N = 11) at dose levels of 0.03, 0.1, 0.3, or 1.0µg/kg/d subcutaneously (SC) for up to 10 days. A dose-dependent increase in platelet count was observed in the patients treated with PEG-rHuMGDF; the onset of this effect was noted on day 6 of treatment and the peak effect, between days 12 and 18. Early thrombocytosis at the 1.0-µg/kg/d dose level resulted in PEG-rHuMGDF treatment being given for a lesser number of days (ie, 6, 7, or 9 days).
In two patients, platelet counts increased to > 1,000 × 109/L without clinical sequelae. One additional patient developed superficial thrombosis, which resolved spontaneously. No changes in leukocyte counts or hematocrit values were observed. Platelet appearance by light and electron microscopy and functional assays was reported to be normal.
In the post-chemotherapy phase of the trial,[64] 31 patients were treated with PEG-rHuMGDF and 10 with placebo. All patients received chemotherapy consisting of carboplatin (Paraplatin), 600 mg/m², and cyclophosphamide (Cytoxan, Neosar), 1,200 mg/m², on day 1. The study drug (PEG-rHuMDGF or placebo) was administered by SC injection from day 2 until either the platelet count rose to 750 × 109/L or the duration of treatment reached 20 days, whichever occurred first. At higher doses (1 to 5 µg/kg), the treatment duration was reduced to 7 days because of recovery thrombocytosis. In addition, all patients received G-CSF, 5 µg/kg/d SC, until neutrophils recovered to a level of 10×109/L.
As compared with placebo, PEG-rHuMGDF enhanced platelet recovery in a dose-related manner. The platelet nadir occurred earlier in the PEG-rHuMGDF-treated group. How-ever, there was no difference in the depth of the nadir. Platelet recovery to baseline level was faster, especially in pa-tients who received pretreatment with PEG-rHuMGDF.
Two patients given PEG-rHuMGDF experienced a thrombotic episode (pulmonary embolism in one patient and thrombophlebitis in the calf in the other). Bruising and bleeding related to thrombocytopenia were infrequent and mild when they occurred. No patient in this trial developed antibodies to PEG-rHuMGDF.
Pegylated recombinant human megakaryocyte growth and development factor was also evaluated in a randomized, placebo-controlled, dose-escalation study in patients with lung cancer treated with carboplatin, dosed to an area under the curve (AUC) of 9 (calculated using the Calvert formula), and paclitaxel (Taxol), 175 mg/m².[65] Following chemotherapy, 37 patients received PEG-rHuMGDF (at dose levels of 0.03, 0.1, 0.3, 1.0, 3.0, or 5.0 µg/kg/d SC) for 3 to 16 days and 10 patients received placebo. The median nadir platelet count was 188,000/mm³ in the PEG-rHuMGDF-treated patients, as compared with 111,000/mm³ in the placebo-treated patients. The time to recovery of baseline platelet count was 1 week earlier in the PEG-rHuMGDF-treated patients. Interestingly, no dose-response to PEG-rHuMGDF was observed. No effects were seen on white blood cell counts or hematocrit values.
Two patients given PEG-rHuMGDF experienced a thrombotic episode. No patient developed antibodies to PEG-rHuMGDF.
Recently, a randomized, placebo-controlled dose-scheduling trial of PEG-rHuMGDF with G-CSF was carried out in non-small-cell lung cancer patients treated with paclitaxel (175 mg/m²) and carboplatin (dosed to an AUC of 11). In this trial,[66] patients were randomized to receive PEG-rHuMGDF at a dose of 2.5 or 5 µg/kg for 1, 3, or 7 days. The platelet nadir was higher in the group treated with PEG-rHuMGDF than in the placebo group (89 vs 27 ×109/L in cycle 1) and the transfusion requirement was lower (17% vs 64% in the first two cycles). However, in the later cycles thrombocytopenia became dose-limiting in all treatment groups. More importantly, three patients developed neutralizing antibodies that were associated with thrombocytopenia. Further follow-up and reporting on these observations would help determine the clinical impact of these findings.
Clinical Trials of rhTPO
Phase I/II clinical trials of rhTPO have been carried out in cancer patients both prior to and following chemotherapy.[67] The design of the initial trial in patients with sarcoma was based on preclinical studies in which a single dose of rhTPO was sufficient to decrease the nadir and accelerated platelet recovery in mice rendered pancytopenic by sublethal radiation and chemotherapy. More prolonged treatment of up to 8 days did not provide additional advantage and was accompanied by a recovery thrombocytosis.
As a result of these observations, rhTPO was given as a single dose prior to chemotherapy to sarcoma patients who were at high risk of developing chemotherapy-induced thrombocytopenia. The initial results of the prechemotherapy phase of this trial were recently reported.[66] Treatment with a single dose of rhTPO (0.3, 0.6, 1.2, or 2.4 µg/kg given intravenously [IV]) resulted in a dose-dependent rise in circulating platelet count (1.3- to 3.6-fold) in all patients.
The rise in platelet count was seen as early as day 4, suggesting that rhTPO enhanced maturation of megakaryocytes and/or release of platelets. The peak effect was seen on a median of day 12 and the duration of response was sustained, such that day 21 counts were still higher than baseline values. Such a prolonged response to a single dose can be explained by multiple contributing factors, including the long circulating half-life of rhTPO (18 to 32 hours); the proliferative effects of rhTPO at the progenitor-cell level, along with the maturation effect on megakaryocytes; and the 9- to 10-day lifespan of platelets.
The proliferative effect of rhTPO was supported by the in vivo biological effects observed in this trial. Recombinant human thrombopoietin increased both the frequency and proliferative rate of progenitor cells of multiple cell lineages and increased the number of bone marrow CD34+ and CD41+ cell populations and immature megakaryocytes (2N-8N). The maturational effect was supported by an increase in the size and ploidy (64N) of bone marrow megakaryocytes.
Interestingly, a single dose of rhTPO also had a potent effect on mobilization of peripheral blood progenitor cells.[68] Despite this multilineage effect at the progenitor-cell level, the peripheral effects of rhTPO in patients with normal hematopoietic functions were limited to platelet lineage.
Early results of the post-chemotherapy phase of this trial demonstrate the potential for rhTPO to attenuate chemotherapy-induced thrombocytopenia.[67] The observation that the peak biological effect of rhTPO occurs between days 10 to 15 and the fact that the chemotherapy nadir effect may also be seen during this interval suggest that the timing of rhTPO administration in relation to chemotherapy may be important. Ongoing trials are attempting to optimize the dose and schedule of rhTPO in this setting.
A second trial of rhTPO in the chemotherapy setting has been carried out in patients with gynecologic malignancy receiving high-dose carboplatin.[69] In this trial, rhTPO was given as a single dose by the SC route 3 weeks prior to chemotherapy. The first carboplatin cycle (at a dose calculated to achieve an AUC of 11) was administered without rhTPO. The second cycle of carboplatin (at the same dose) was administered 3 weeks later to be followed by rhTPO. Because the nadir induced by carboplatin is delayed (approximately day 16), four doses of rhTPO were used over 8 days (every other day). The nadir platelet count was higher (52 vs 20× 10³/mm³) and duration of thrombocytopenia (< 50 ×10³/mm³) was shorter (3 vs 6 days) in cycle 2 as compared to cycle 1 when rhTPO was given for four doses after carboplatin in cycle 2 (N = 22).
Results of this trial show that rhTPO is effective in attenuating both the degree and duration of thrombocytopenia associated with high-dose carboplatin. In addition, when used as secondary prophylaxis, rhTPO also reduced the need for platelet transfusions in this group of patients at high risk for severe thrombocytopenia. No serious adverse events related to rhTPO or to thrombocytosis were observed in these trials.
Potential Clinical Applications of Thrombopoietin
The results of preclinical and early clinical studies demonstrate that thrombopoietin is a potent stimulator of platelet production in vivo. Given its lineage-dominant biological effects, thrombopoietin has a very favorable toxicity profile. In this respect, it compares favorably with other thrombopoietic cytokines, which mediate a multitude of biological effects and often are associated with many side effects. These observations suggest a potential clinical utility of thrombopoietin in several clinical settings.
Cancer Patients Receiving Myelosuppressive Therapy
In a manner analogous to other lineage-dominant hematopoietic growth factors, thrombopoietin will likely attenuate thrombocytopenia induced by chemotherapy and/or radiotherapy and accelerate platelet recovery. Results of early clinical trials using a moderately myelosuppressive regimen demonstrate the therapeutic potential of thrombopoietin in this clinical setting.
However, several end points of benefit will have to be considered in evaluating the efficacy of this agent. Since major bleeding or death are rare complications of chemotherapy, a reduction in or prevention of prophylactic platelet transfusions and, thus, a decrease in patient inconvenience and health-care costs would be the primary end points for assessing efficacy.
It should also be recognized that whereas severe thrombocytopenia requiring platelet transfusions occurs routinely in intensive treatment of leukemia and pediatric malignancies and in the BMT setting, it is less frequent and more of a cumulative problem with most chemotherapeutic regimens used to treat solid tumors. In that regard, the availability of appropriate target cells (ie, responsive progenitor cells) for thrombopoietin to act on is critical. Thus, a paucity of healthy stem cells may limit the activity of this cytokine in patients with compromised bone marrow.
The clinical utility of thrombopoietin in patients who have undergone myeloablative treatment and BMT may also depend on the available pool of responsive precursor cells. However, in those who have received transplants of peripheral blood progenitor cells, which enhance platelet recovery and thus likely contain progenitors of megakaryocyte lineage, thrombopoietin may further attenuate the period of severe thrombocytopenia.
Bone Marrow Failure States
Patients with bone-marrow failure related to intrinsic disease processes, such as aplastic anemia and myelodysplastic syndromes, have elevated endogenous thrombopoietin levels together with low circulating platelet counts. The response of these patients to pharmacologic doses of thrombopoietin would be limited, therefore, by the reserve of responsive stem cells. However, similar to erythropoietin, which is effective in approximately 20% of anemic patients with myelodysplastic syndromes, thrombopoietin may be effective in some patients with these disorders.
Transfusion Medicine
Given its potent biologic effects and possibly minimal toxicity, thrombopoietin will likely be used in transfusion medicine for the acquisition of platelets from normal donors or patients (autologous donation) to be stored and used as needed. Administration of a single dose of thrombopoietin prior to chemotherapy could be used to raise platelet number severalfold. These platelets could then be stored for long periods using cryopreservation techniques.[70,71] This may reduce the problem of alloimmunization with multiple donor exposures and may provide autologous cellular support when, after multiple cycles of chemotherapy, compromised marrow may not be responsive to cytokine treatment in vivo.
Other Potential Uses
Thrombopoietin may be potentially useful in other conditions that produce thrombocytopenia, such as immune thrombocytopenic purpura, human immunodeficiency virus (HIV) infection and/or treatment with antiretroviral agents, cardiac surgery, liver failure, and, perhaps, other diseases and disorders of platelet productions.[72] In patients with immune thrombocytopenic purpura, the level of thrombopoietin is inadequate, and therefore replacement may alleviate the abnormality. In patients with HIV infection, immune thrombocytopenic purpura is also seen, as well as the thrombocytopenia associated with antiretroviral therapy. As erythropoietin has been shown to ameliorate anemia in HIV-infected patients, so may thrombopoietin be useful in treating thrombocytopenia in these patients.
The loss of liver mass[73] or function is a known etiology of thrombocytopenia that may stem from insufficient thrombopoietin levels secondary to loss of production. This condition may respond to replacement therapy.
In addition, patients undergoing surgery who are at increased risk for thrombocytopenia and bleeding complications may benefit from presurgical stimulation of platelet production and storage for postoperative use, in a manner similar to autologous-packed red blood cell collections.
Potential Safety Concerns With Thrombopoietin
Although thrombopoietin has been well tolerated in early clinical trials, more experience with long-term follow-up is necessary to fully assess the safety profile of this new agent.
Thrombosis
One concern that relates to the rapid generation of young platelets with potential for activation is an increased risk of thrombosis. In vitro studies suggest that thrombopoietin can increase the responsiveness of platelets to subthreshold levels of agonists.[74,75] Given the normal morphology and aggregation function of platelets following thrombopoietin treatment and the transient high platelet count following recovery from chemotherapy, such a risk should be low. However, thrombotic episodes have been reported in a few patients treated with thrombopoietin in initial clinical trials.[64,65] Further experience will help determine the magnitude of this risk.
Immunogenicity
One of the potential concerns with the use of recombinant molecules is the risk of development of neutralizing antibodies. To date, the reported experience with both forms of thrombopoietin molecules is limited. While no neutralizing antibodies have been observed in three reported clinical trials with either of these molecules, more recently, neutralizing antibodies have been observed in a few cancer patients who received PEG-rHuMGDF. This is a potentially serious concern in light of the resulting thrombocytopenia. Further, larger experience with both of these agents will be necessary to determine the nature and the extent of the problem.
Bone Marrow Fibrosis
Another potential risk of thrombopoietin may be fibrosis in the bone marrow induced by the marked increase in bone-marrow megakaryocytes and increased release of platelet-derived growth factors. In thrombopoietin-overexpressing mice (induced by retroviral-mediated gene transfer), marked stimulation of megakaryocytopoiesis, marrow fibrosis, osteosclerosis, and splenomegaly have been observed.[76] These findings, which may be related to cytokines produced by megakaryocytes and platelets, recede when the factor is withdrawn.
In human trials, short-term follow-up bone marrow examinations have shown no evidence of increased fibrosis. In clinical situations in which thrombopoietin is used over the short term, it is unlikely that such an overexposure to high concentration of thrombopoietin will occur. Nevertheless, careful long-term follow-up is needed to assess this risk.
Stimulation of Tumor Cells
Finally, there is a potential concern that thrombopoietin may stimulate the growth of malignant cells. The receptor for thrombopoietin, c-Mpl, is expressed primarily on hematopoietic cells. A large number of nonhematopoietic solid tumors have been analyzed and were not found to express c-Mpl.[77]
Also, c-Mpl receptor expression was not increased in bone-marrow cells from several patients with acute lymphocytic leukemia, chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, or non-Hodgkin’s lymphoma.[78] However, about half of patients with acute myeloid leukemia (26 of 51 patients) and myelodysplastic syndrome (5 of 11 patients) showed an increase in c-Mpl receptors.[76] The expression of c-Mpl in acute myeloid leukemia was associated with a poor prognosis and lower response to chemotherapy.[75] These findings suggest the need for caution regarding the use of thrombopoietin in patients with acute myeloid leukemia.
However, since the induction treatment for leukemia is routinely associated with severe thrombocytopenia, which may benefit from an agent that can enhance platelet recovery and reduce platelet transfusions, carefully designed clinical trials are needed to evaluate the role of thrombopoietin in this setting. It should be recognized that similar concerns were raised about the use of myeloid growth factors in acute myeloid leukemia because of the expression of receptors for G-CSF and GM-CSF on some leukemic cells. Thus far, clinical experience with these growth factors has not shown any overtly detrimental effect on response rate or leukemia regrowth.
Conclusions
The last two decades have witnessed the isolation, purification, and cloning of several hematopoietic growth factors that regulate hematopoiesis and production of mature blood cells. The clinical development of these cytokines has led to a new class of therapeutic agents that have been extensively utilized in the management of cancer patients undergoing myelosuppressive therapy.
With the discovery of thrombopoietin, the long-sought-after lineage-specific cytokine has been identified. The in vitro properties and in vivo biology of thrombopoietin indicate that it is, indeed, a primary regulator of platelet production in vivo. Early clinical studies suggest that this potent stimulator of platelet production will likely be useful in the prevention and management of thrombocytopenia observed in hematology and oncology practices.
However, most recently, clinical trials of PEG-rHuMGDF (the pegylated, truncated version) have been discontinued based on the development of neutralizing antibodies in some cancer patients as well as in normal donors. No neutralizing antibodies have been reported thus far with rhTPO (the full-length molecule), and further evaluation of the safety and activity of rhTPO is ongoing.
Carefully designed clinical studies are needed to fully assess the safety profile and clinical merits of this thrombopoietic agent. These trials will have to pay careful attention to the following issues: (1) the need for platelet transfusions (ie, the platelet level associated with significant risk for bleeding complications) and most clinicians' level of comfort in administering prophylactic transfusions using the lower trigger point [10,000 vs 20,000/mm3]; (2) the need for maintaining dose intensity of chemotherapy that requires use of a thrombopoietic agent; (3) and the impact of thrombopoietin administration on health-care costs and patients’ quality of life.
References:
1. Elias A, Ryan L, Aisner J, et al: Mesna, Doxorubicin, ifosfamide, dacarbazine (MAID) regimen for adults with advanced sarcoma. Semin Oncol 17(2 suppl 4):41-49, 1990.
2. Schutte J, Mouridsen H, Stewart W, et al: Ifosfamide plus doxorubicin in previously untreated patients with advanced soft tissue sarcoma. Cancer Chemother Pharmacol  31(suppl 2):204-209, 1993.
3. Hill M, Macfarlane V, Moore J, et al: Taxane/platinum/anthracycline combination therapy in advanced epithelial ovarian cancer. Semin Oncol 24(1; suppl 2):34-37, 1997.
4. Veldhuis G, Williams P, Beijnew J, et al: Paclitaxel, ifosfamide and cisplatin with granulocyte colony-stimulating factor or recombinant human interleukin 3 and granulocyte colony stimulating factor in ovarian cancer: A feasibility study. Br J Cancer 75(5):703-709, 1997.
5. Williams S, Birch R, Einhorn L, et al: Treatment of disseminated germ-cell tumor with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med 316:1435-1440, 1987.
6. Loehrer P, Lauer R, Roth B, et al: Salvage therapy in recurrent germ cell cancer: Ifosfamide and cisplatin plus either vinblastine or etoposide. Ann Intern Med 109:540-546, 1988.
7. Krigel R, Palackdharry C, Padavic K, et al: Ifosfamide, carboplatin and etoposide plus granulocyte colony-stimulating factor: A phase I study with apparent activity in non-small-cell lung cancer. J Clin Oncol 12:1251-1258, 1994.
8. Bonadonna G, Valagussar P, Santos A: Alternating non-cross resistant combination chemotherapy or MOPP in stage IV Hodgkin’s disease. Ann Intern Med 104:739-746, 1986.
9. Goss P, Shepherd F, Scott G, et al: Dexamethasone/ ifosfamide/ cisplatin/etoposide (DICE) as therapy for patients with advanced refractory non-Hodgkin’s lymphoma: Preliminary report of a phase II study. Ann Oncol 2(suppl 1):43-46, 1991.
10. Dana B, Dahlberg S, Miller T, et al: M-BACOD treatment for intermediate and high-grade malignant lymphoma: A Southwest Oncology Group phase II trial. J Clin Oncol 8:1155-1162, 1990.
11. Pruesser P, Wilke H, Achterrath W, et al: Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol 7(9):1310-1317, 1989.
12. Bernstein SH, Nademanee A, Vose J, et al: A multicenter study of platelet recovery and utilization in patients after myeloablative therapy and hematopoietic stem-cell transplantation: Cytokine growth factors in hematology and oncology. Blood 91(9):3509-3517, 1998.
13. Wallace EL, Churchill WH, Suregenor DM, et al: Collection and transfusion of blood and blood components in the United States, 1992. Transfusion 35:802-812, 1995.
14. Heyman MR, Schiffer CA: Platelet transfusion therapy for the cancer patient. Semin Oncol 17:198-209, 1990.
15. Malone D, Sullivan S, Black D, et al: The cost of treating chemotherapy-induced thrombocytopenia (abstract). Proc Am Soc Clin Oncol 14:305, 1995.
16. Kuter DJ: The physiology of platelet production. Stem Cells 14(suppl 1):88-101, 1996.
17. Gewirtz A: Megakaryocytopoiesis: The state of the art. Thromb Haemost 74(1):204-209, 1995.
18. Hoffman R, Murray L, Young J, et al: Hierarchical structure of human megakaryocyte progenitor cells. Stem Cells 14(suppl l):75-81, 1996.
19. Kelemen E, Cserhati I, Tanos B: Demonstration and some properties of human thrombopoietin in thrombocythaemic sera. Acta Haematol 20:350-355, 1958.
20. Vignon I, Mornon J, Cocault L, et al: Molecular cloning and characterizations of Mpl, the human homolog of the v-mpl oncogene: Identification of a member of the hematopoietic growth factor superfamily. Proc Natl Acad Sci USA 89:5640, 1992.
21. Wendling F, Varlet P, Charon M, et al: A retrovirus complex inducing an acute myeloproliferative leukemia disorder in mice. Virology 149:242, 1986.
22. Souyri M, Vigon I, Penciolelli JF, et al: A putative truncated cytokine receptor gene transduced by the myeloproliferative leukemia virus immortalizes hematopoietic progenitors. Cell 63:1137-1147, 1990.
23. Methia N, Louache F, Vainchenker W, et al: Oligodeoxynucleotides antisense to the proto-oncogene c-Mpl specifically inhibits in vitro megakaryocytopoiesis. Blood 82:1395-1401, 1993.
24. Bartley TD, Bogenberg J, Hunt P, et al: Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell 77:1117-1124, 1994.
25. de Savage FJ, Hass PB, Spencer SD, et al: Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 369:533-538, 1994.
26. Kuter DJ, Beeler DL, Rosenberg RD: The purification of megapoietin: A physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci USA 91:11104-11108, 1994.
27. Lok S, Kaushansky K, Holly RD, et al: Cloning and expression of murine thrombopoietin CDNA and stimulation of platelet production in vivo. Nature 369:565-568, 1994.
28. Sohma Y, Akahori H, Seki N, et al: Molecular cloning and chromosomal localization of the human thrombopoietin gene. Federation of European Biochemical Societies Lett 353:57-61, 1994.
29. Wendling F, Maraskovsky E, Debili N, et al: c-Mpl ligand as a humoral regulator of megakaryopoiesis. Nature 369:571-574, 1994.
30. Chang M, McNinch J, Basu R, et al: Cloning and characterization of the human megakaryocyte growth and development factor (MGDF) gene. J Biol Chem 270(2):511-514, 1995.
31. Foster D, Lok S: Biological roles for the second domain of thrombopoietin. Stem Cells 14(suppl 1):102-107, 1996.
32. Debili N, Wendling F, Cosman, et al: The Mpl receptor is expressed in the megakaryocyte lineage from later progenitors to platelets. Blood 85:391, 1995.
33. Le Conniat M, Souyri M, Vigon I, et al: The human homolog of the myeloproliferative leukemia virus maps to chromosome band 1p34. Hum Genet 83:194-196, 1989.
34. Gurney A, de Sauvage F: Dissection of c-Mpl and thrombopoietin function: Studies of knockout mice and receptor signal transduction. Stem Cells 14(suppl 1):116-123, 1996.
35. Kaushansky K, Lok S, Holly RD, et al: Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 369:568-571, 1994.
36. Broudy VC, Lin NL, Kaushansky K: Thrombopoietin (c-Mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood 85:1719, 1995.
37. Kaushansky K, Broudy VC, Lin N, et al: Thrombopoietin, the Mpl-ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci USA 92:3234-3238, 1995.
38. Ziegler FC, de Sauvage F, Widmer HR, et al: In vitro megakaryocytopoietic and thrombopoietic activity of c-Mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood 84:4045, 1994.
39. Zucker-Franklin D: Megakaryocyte and platelet structure in thrombocytopoiesis: The effect of cytokines. Stem Cells 14(suppl 1):1-17, 1996.
40. Kaushansky K: Thrombopoietin: The primary regulator of platelet production. Blood 86(2):419-431, 1995.
41. Kobayashi M, Layer HJ, Kato T, et al: Recombinant human thrombopoietin (Mpl ligand) enhances proliferation of erythroid progenitors. Blood 86:2494-2499, 1995.
42. Sitnicka B, Lin N, Priestley GV, et al: The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood 87:87:4998-5005, 1996.
43. Young JC, Bruno B, Luens KM, et al: Thrombopoietin stimulates megakaryocytopoiesis, myelopoiesis and expansion of CD34+Thy-l+Lin primitive progenitor cells. Blood 88:1619-1631, 1996.
44. Ramsfjell V, Borge OJ, Vieby OP, et al: Thrombopoietin, but not erythropoietin, directly stimulates multi-lineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokines: Distant interactions with the ligands for c-kit and FLT3. Blood 88(12):4481- 4492, 1996.
45. Kuter DJ, Rosenberg RD: The appearance of a megakaryocyte growth promoting activity, megapoietin, during acute thrombocytopenia in the rabbit. Blood 84:1464-1472, 1995.
46. Cohen-Solal K, Villeval J-L, Titeux M: Constitutive expression of Mpl ligand transcripts during thrombocytopenia or thrombocytosis. Blood 88:2578-2584, 1996.
47. Nichol JL, Hokom MM, Homkohl A, et al: Megakaryocyte growth and development factor: Analyses of in vitro effects on human megakaryopoiesis and endogenous serum levels during chemotherapy-induced thrombocytopenia. J Clin Invest 95:2973-2979, 1995.
48. Meng YG, Martin TG, Peterson ML: Circulating thrombopoietin concentrations in thrombocytopenic patients, including cancer patients following chemotherapy, with or without peripheral blood progenitor cell transplantation. Br J Haematol 95(3):535-544, 1996.
49. Emmons R, Reid DM, Cohen RL: Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood 87:4068-4071, 1996.
50. Gurney AL, Caver-Moore K, de Sauvage FJ, et al: Thrombocytopenia in c-Mpl-deficient mice. Science 265:1445-1447, 1994.
51. de Sauvage F: Physiologic regulation of early and late stages of megakaryocytosis by thrombopoietin. J Exp Med 183:651-656, 1996.
52. Alexander WS, Roberts AW, Nicola NA: Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietin receptor c-Mpl. Blood 87:2162-2170, 1996.
53. Carver-Moore K, Broxmeyer HE, Luoh SM: Low levels of erythroid and myeloid progenitors in thrombopoietin and c-Mpl-deficient mice. Blood 88: 803-808, 1996.
54. Gurney A, deSauvage F: Dissection of c-Mpl and thrombopoietin function: Studies of knockout mice and receptor signal transduction. Stem Cells 14(suppl 1):116-123, 1996.
55. Fielder P, Gurney A, Stefanich E, et al: Regulation of thrombopoietin levels by c-Mpl-mediated binding to platelets. Blood 87:2154-2161, 1996.
56. Tahara T, Usuki K, Sato H, et al: A sensitive sandwich ELISA for measuring thrombopoietin in human serum: Serum thrombopoietin levels in healthy volunteers and in patients with haematopoietic disorders. Br J Haematol 93:783-788, 1996.
57. Kaushansky K, Lin N, Grossman A, et al: Thrombopoietin expands erythroid, granulocyte-macrophage and megakaryocytic progenitor cells in normal myelosuppressed mice. Exp Hematol 24(2):265-269, 1996.
58. Ulich TR, del Castillo J, Yin S, et al: Megakaryocyte growth and development factor ameliorates carboplatin-induced thrombocytopenia in mice. Blood 86:971-976, 1995.
59. Hokom MM, Lacey D, Kinstler O, et al: Pegylated megakaryocyte growth and development factor abrogates the lethal thrombocytopenia associated with carboplatin and irradiation in mice. Blood 86:4486-4492, 1995.
60. Thomas GR, Thibodeaux H, Errett CJ, et al: In vivo biological effects of various forms of thrombopoietin in a murine model of transient pancytopenia. Stem Cells 1:245-266, 1996.
61. Farase A, Hunt P, Boone T, et al: Recombinant human megakaryocyte growth and development factor stimulates thrombocytopoiesis in normal nonhuman primates. Blood 86:54-59, 1995.
62. Nichol J: Preclinical biology of megakaryocyte growth and development factor: A summary. Stem Cells 14(suppl 1):48-52, 1996.
63. Basser R, Rasko J, Clarke K, et al: Thrombopoietic effects of pegylated recombinant human megakaryocyte growth and development factor (PEG-HuMGDF) in patients with advanced cancer. Lancet 348:1279-1281, 1996.
64. Basser R, Rasko J, Clarke K, et al: Randomized, blinded, placebo-controlled phase I trial of pegylated recombinant human megakaryocyte growth and development factor with filgrastim after dose-intensive chemotherapy in patients with advanced cancer. Blood 89:3118-3128, 1997.
65. Fanucchi M, Glaspy J, Crawford J, et al: Effects of polyethylene glycol-conjugated recombinant human megakaryocyte growth and development factor on platelet counts after chemotherapy for lung cancer. N Engl J Med 336:404-409, 1997.
66. Crawford J, Glaspy J, Belani C, et al: A randomized, placebo-controlled, blinded, dose scheduling trial of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) with filgrastim support in non-small-cell lung cancer (NSCLC) patients treated with paclitaxel and carboplatin during multiple cycles of chemotherapy (abstract). Proc Am Soc Clin Oncol 17:73a, 1998.
67. Vadhan-Raj S, Patel S, Broxmeyer HE, et al: Phase I-II investigation of recombinant human thrombopoietin (rhTPO) in patients with sarcoma receiving high-dose chemotherapy with Adriamycin and ifosfamide. Blood 88:448a, 1996.
68. Vadhan-Raj S, Murray L, Bueso-Rumos C, et al: Stimulation of megakaryocyte and platelet production by a single dose of recombinant human thrombopoietin in patients with cancer. Ann Intern Med 126:673-681, 1997.
69. Vadhan-Raj S, Verschraegen C, McGarry L, et al: Recombinant human thrombopoitin (rhTPO) attenuates high-dose carboplatin (C)-induced thrombocytopenia in patients with gynecologic malignancy (abstract). Blood 90:580a, 1997.
70. Connors J, Curie LM, Allan H, et al: Recovery of in vitro functional activity of platelet concentrates stored at 4°C and treated with second-messenger effectors. Transfusion 36:691-698, 1996.
71. Currie LM, Vadhan-Raj S, and Connor J: Cryopreservation of platelets from recombinant human thrombopoietin (rhTPO)-treated donors using thrombosol and 2% DMSO. J Am Soc Hematol 88(suppl 1):1316, 1996.
72. Kuter D: Thrombopoietin: Biology, clinical applications, role in the donor setting. J Clin Apheresis 11(3):149-159, 1996.
73. Siemensma NP, Bathal PS, Penington DG: The effect of massive liver resection on platelet kinetics in the rat. J Lab Clin Med 86:817-833, 1975.
74. Ezumi Y, Takayama H, Okuma M: Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2 and STAT3, and enhances agonist-induced aggregation in platelets in vitro. Federation of European Biochemical Societies Lett 3 74:48-52, 1995.
75. Toombs CF, Young CH, Glaspy JA: Megakaryocyte growth and development factor (MGDF) moderately enhances in vitro platelet aggregation. Thromb Res 80:23-33, 1995.
76. Yan X-Q, Lacey D, Fletcher, et al: Chronic exposure to retroviral vector encoded MGDF (mpl-Ligand) induces lineage-specific growth and differentiation of megakarocytes in mice. Blood 88:4025-4033, 1995.
77. Columbyova L, Loda M, Scadden DT: Thrombopoietin receptor expression in human cancer cell lines and primary tissues. Cancer Res 55:3509-3512, 1995.
78. Vigon I, Dreyfus F, Melle J, et al: Expression of the c-Mpl protooncogene in human hematologic malignancies. Blood 82:877-883, 1993.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.