Topical Gel Significantly Improves EGFR Inhibitor-Related Skin Toxicity
All patients who received HT-001 in the phase 2a CLEER-001 trial showed significant skin toxicity improvements by 6 weeks.
In the open-label pharmacokinetics portion of the multi-center phase 2a CLEER-001 trial (NCT05639933), patients received topical treatment with HT-001 2% gel.
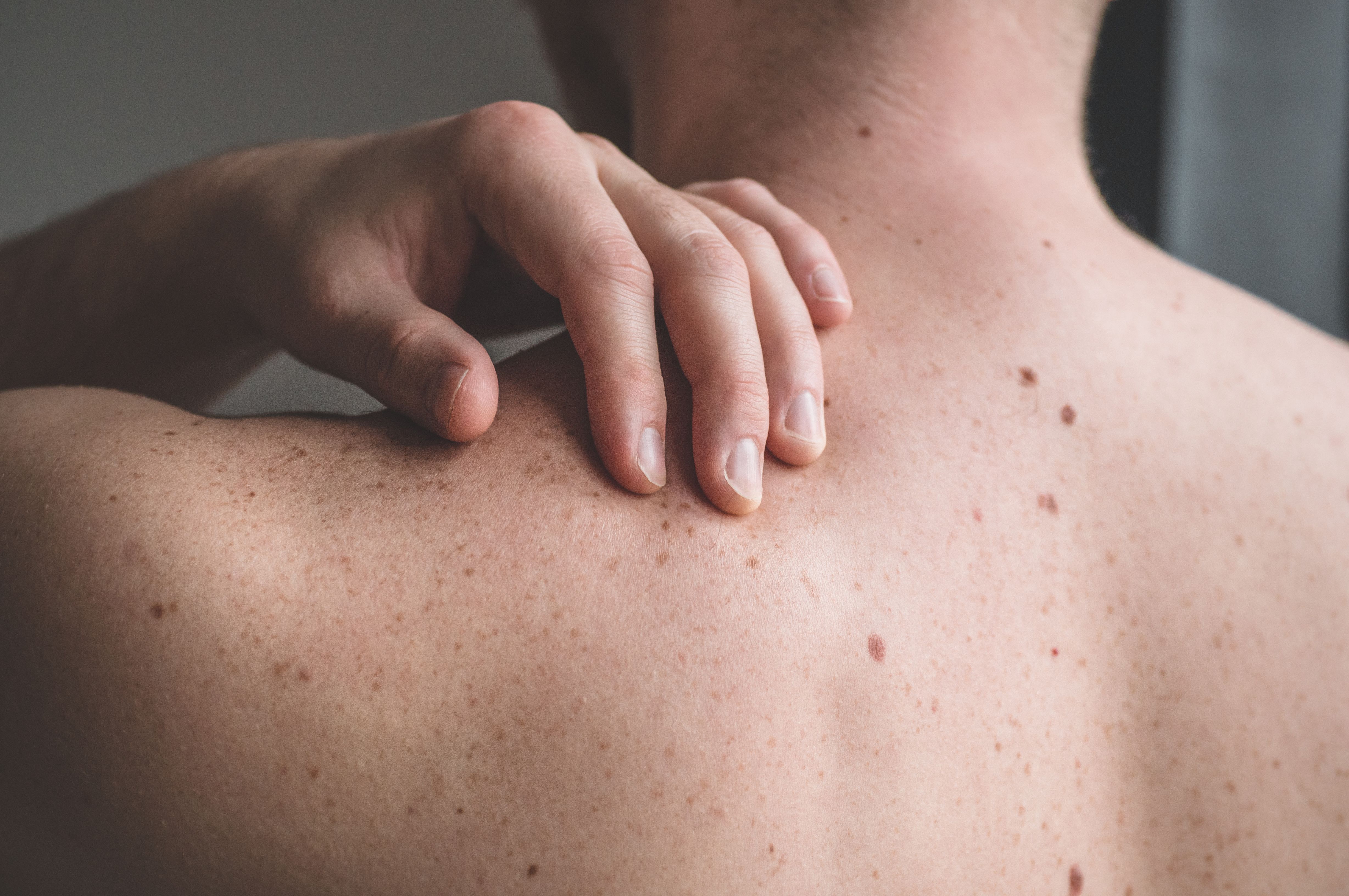
Investigational topical gel HT-001 produced encouraging efficacy and safety outcomes among patients with cancer who experienced skin toxicities associated with prior EGFR inhibitor therapy, according to a press release on findings from the phase 2a CLEER-001 trial (NCT05639933) from developers Hoth Therapeutics, Inc.1
During the open-label portion of the study, HT-001 yielded an Acneiform Rash Investigator Global Assessment Scale (AGIRA) score of 1 or lower among 100% of patients, representing significant improvements in skin toxicity by 6 weeks. Regarding other improvements in quality of life, 66% of patients experienced reductions in pain and itching scores.
Of note, all patients remained on their full dose of EGFR inhibitor therapy following dosing of HT-001, thereby maintaining the full therapeutic efficacy of their anti-cancer therapy. Additionally, investigators reported no treatment-related adverse effects (TRAEs) in the trial.
“These results are a significant milestone, underscoring HT-001's potential to transform patient care by mitigating debilitating skin toxicities while maintaining critical cancer treatments,” Robb Knie, chief executive officer at Hoth Therapeutics, said in the press release.1 “Our data highlight HT-001's strong safety profile and the potential for it to set a new standard of care in this underserved area.”
In the open-label pharmacokinetics portion of the multi-center phase 2a CLEER-001 trial, patients received topical treatment with HT-001 2% gel.2 During the randomized, double-blind portion, patients were randomly assigned to receive HT-001 as 2%, 1%, or 0.5% topical gels or placebo therapy with vehicle gel.
The trial’s primary end points included the proportion of patients with a score of 1 or lower on the ARIGA scale and the pharmacokinetics of HT-001 in terms of area under the curve concentration and peak plasma concentration. Developers designed the proprietary ARIGA scale in collaboration with onco-dermatology team members to precisely measure and assess skin toxicity improvements. Secondary end points included changes in the Pruritus Numeric Rating Scale, changes in the Pain Numeric Rating Scale, changes in acneiform rash severity, time to improvement, time to rescue therapy, EGFR inhibitor dose reductions or discontinuations, and safety and tolerability.
Patients 18 years and older who previously received an approved EGFR inhibitor to treat cancer and symptoms of rash per CTCAE grading and AGIRA scales were eligible for enrollment on the trial. Other requirements for study entry included having an ECOG performance status of 0 to 2, a predicted life expectancy of 3 months or longer, the ability and willingness to attend necessary telehealth and in-person visits, and the willingness to provide informed consent.
Those with severe cutaneous toxicity following EGFR inhibitor therapy or a history of other skin disorders that might confound study results or pose unwarranted risks when administering treatment with HT-001 were ineligible for enrollment on the trial. Patients were also unable to enroll if they had prior aprepitant (Emend) or other neurokinin-1 receptor antagonists within 4 weeks of screening, an active infection or any uncontrolled disease, non-stable escalating doses of topical antibiotics within 2 weeks of screening, non-stable escalating doses of systemic steroids within 2 weeks of screening, or history of hypersensitivity to aprepitant or any component of HT-001.
“These interim findings align with a recent case report of rapid resolution of EGFR [inhibitor]-induced skin conditions using HT-001. As the study progresses, we anticipate further validating these results and are excited about the potential impact HT-001 could have on patient outcomes,” Knie concluded.1
References
- Hoth Therapeutics achieves breakthrough in phase 2a trial: HT-001 delivers 100% success in combating cancer treatment skin toxicities. News release. Hoth Therapeutics, Inc. January 7, 2025. Accessed January 9, 2025. https://tinyurl.com/4k435ytp
- Study to investigate the efficacy, safety, and tolerability of topical HT-001 for the treatment of skin toxicities associated with epidermal growth factor receptor inhibitors (CLEER). ClinicalTrials.gov. Updated December 24, 2024. Accessed January 9, 2025.
How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.
How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.