Treating Advanced Breast Cancer in the Older Woman
As half of all breast cancers occur in patients beyond the age of 65 and a quarter beyond the age of 75, a significant number of patients with metastatic breast cancer are elderly. New hormonal therapies, such as aromatase inhibitors, appear to have favorably improved the survival of these patients. Side effects such as osteoporosis or cognitive issues appear manageable. Information specific to elderly patients has recently emerged in the field of chemotherapy for metastatic breast cancer. This article reviews data on anthracyclines, taxanes, capecitabine (Xeloda), gemcitabine (Gemzar), trastuzumab (Herceptin), and bevacizumab (Avastin). For most patients in this setting, sequential single-agent chemotherapy appears at this time to be the preferred course of treatment.
As half of all breast cancers occur in patients beyond the age of 65 and a quarter beyond the age of 75, a significant number of patients with metastatic breast cancer are elderly. New hormonal therapies, such as aromatase inhibitors, appear to have favorably improved the survival of these patients. Side effects such as osteoporosis or cognitive issues appear manageable. Information specific to elderly patients has recently emerged in the field of chemotherapy for metastatic breast cancer. This article reviews data on anthracyclines, taxanes, capecitabine (Xeloda), gemcitabine (Gemzar), trastuzumab (Herceptin), and bevacizumab (Avastin). For most patients in this setting, sequential single-agent chemotherapy appears at this time to be the preferred course of treatment.
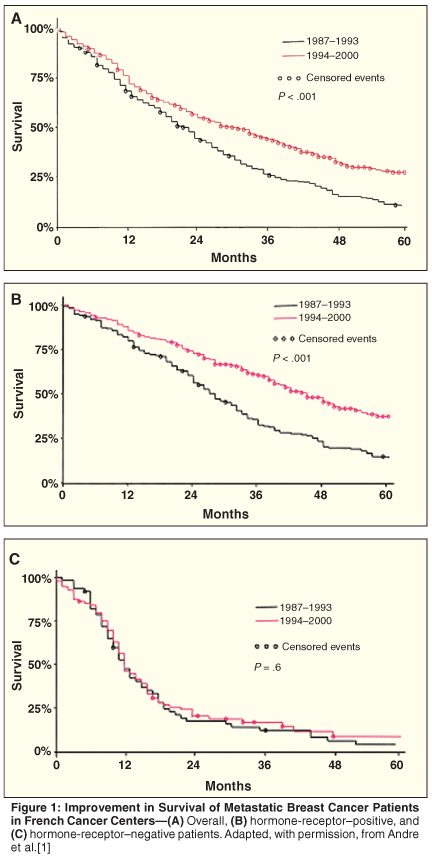
There is good news for metastatic breast cancer patients: Their survival is increasing-from 27% at 3 years in the early 1990s to 44% in the late 1990s, if they are metastatic at presentation (Figure 1).[1] Although these retrospective data do not allow a clear assessment of causality, it is a reasonable hypothesis that the answer lies in the dramatic increase of treatment options available for this disease and their more widespread application. This article will review to what extent this is also true for the elderly, and how well these drugs are suited to this population.
As age advances, the proportion of aggressive breast cancers with either high-grade, estrogen-receptor (ER)/progesterone-receptor (PR) negativity, or HER2 overexpression decreases. However, such tumors can still develop even in women who are in their 90s. Although these types of tumors pose a higher risk of relapse, even ER/PR-positive, HER2-negative, and lower-grade tumors can reoccur. In such cases, hormonal therapy should be considered.
Hormonal Therapy
The improvement in survival during the 1990s pertains mostly to hormone receptor-positive patients, which is good news for older patients and is likely due in large part to the arrival of the aromatase inhibitors in the armentarium. Antiestrogen therapy has few side effects, but two of them are somewhat more relevant to the elderly than to the young.
One issue is the risk of osteoporosis with aromatase inhibitors, an issue that was primarily raised in the adjuvant setting.[2] However, since certain women with good-prognosis metastatic cancer may undergo years of treatment, the issue should be kept in mind. A pair of large studies Z-FAST and ZO-FAST (Zometa/-Femara Adjuvant Synergy Trials)-are under way to assess whether zoledronic acid (Zometa) can prevent this bone loss. If the results are positive, it will be reassuring news for women with metastatic breast cancer, since the oncologic treatment itself often includes bisphosphonates.
The other potential problem is the cognitive impact of hormonal therapy. Much has been published about this issue, with often inconclusive results. A recent British study comparing patients enrolled in the ATAC trial (Arimidex, Tamoxifen, Alone or in Combination) to untreated controls with a neuropsychological battery found a similar negative impact on cognitive function for anastrozole (Arimidex), tamoxifen, or both agents combined.[3] The effect consisted mainly of a mild decrease in processing speed and verbal memory. The history of the cognitive impact of hormonal therapy, from estrogen replacement to antiestrogens, is a long and confused one. If there is any impact, it appears to be minor and should not preclude treatment.
Chemotherapy
Combination vs Single Agent
The debate over combination chemotherapy vs sequential single agents for metastatic breast cancer patients is ongoing. Many studies have failed to show a survival advantage for combination regimens. Many studies also fail to analyze the contribution of the trial agent given subsequently in the noncombination arm. Certain combination regimens, such as docetaxel (Taxotere) and capecitabine (Xeloda), have shown a survival advantage over single agents, such as docetaxel alone.[4] It should be noted, however, that in the latter study, diarrhea was a prominent problem affecting most of the patients. Furthermore, the doses of capecitabine used have been proven too toxic for older women. Whether this agent's effectiveness is maintained at lower doses is unclear.
Another example is the combination of carboplatin, paclitaxel, and trastuzumab (Herceptin) over paclitaxel and trastuzumab. Time to disease progression was increased from 6.8 to 11.9 months (P = .006), and overall survival was 33.3 and 42.1 months, respectively (P = .27). Although the difference in survival was not significant, it was greater in HER2 3+ disease, and only 196 patients were included in the study.[5] Moreover, the study focused on younger women. Although HER2 positivity occurs less frequently in the elderly, its presence is still possible in this population. In the author's experience, the oldest patient she treated with a trastuzumab-containing regimen was 91 years old.
Carboplatin and paclitaxel, notably if given on a weekly basis, has been shown to be a tolerable regimen in the elderly. When measured with a geriatric evaluation, patients treated with weekly paclitaxel or carboplatin/paclitaxel for various cancers showed improvements in activities of daily living (58%), instrumental activities of daily living (75%), pain (79%), energy (86%), and quality of life (76%).[6] Therefore, such a combination is a reasonable one in a woman with this type of aggressive breast cancer.
On the other hand, a modern single-agent therapy might be better than an older combination in this setting. Docetaxel alone provided a better survival than mitomycin and vinblastine.[7] In this author's opinion, for HER2-negative tumors in the elderly, we lack a clear demonstration that multiagent chemotherapy offers more than a higher initial response rate when compared to sequential single modern agents. Although some phase III studies have reported positive results, they have not been reproduced, and given the number of studies conducted in women with metastatic breast cancer, it is difficult to assess whether the result is representative, or whether we have the 1 in 20 studies that will be positive with a P < .05 just by chance. The same precautionary comment applies to studies comparing single agents with each other.
Tolerance in General
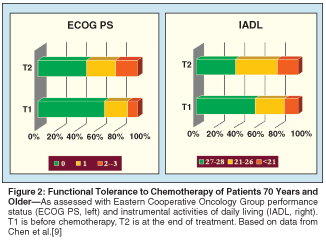
With palliative-intent chemotherapy, one has to ask oneself whether the benefits outweigh the harms. How well is chemotherapy tolerated? For all chemotherapy regimens combined, cancer patients 70 and older have around a 50% risk of experiencing either a grade 3 or 4 nonhematologic or a grade 4 hematologic side effect (National Cancer Institute Common Toxicity Criteria [NCI-CTC]).[8] From a functional point of view, however, these side effects appear to be well tolerated, with three-quarters of patients remaining able of normal or near normal activity (Figure 2).[9] Furthermore, that study involved all types of chemotherapy, including CHOP (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], prednisone). Chemotherapy for metastatic breast cancer tends to be on the low side of the toxicity spectrum.[6]
What is the cognitive impact of chemotherapy in older women? Few answers are available, as most cognitive studies have been conducted in younger cohorts of women. However, a recent German study addressed the question.[10,11] With an age cutoff set at 60 years, the investigators found that although elderly patients experience more cognitive side effects in the first few days after initiating chemotherapy, there was no difference at 6 months in the cognitive impact of chemotherapy between young and old subjects. In fact, both groups improved. An American study assessed patients 65 years and older receiving various adjuvant chemotherapies for breast cancer. At 6 months, 48% had no change in cognitive function, 41% worsened, and 11% improved.[12]
Review of Individual Drugs
In the section below, I will review recent data and experience with agents frequently used to treat metastatic breast cancer.
Anthracyclines
Anthracyclines are increasingly used in the adjuvant treatment of breast cancer in the elderly.[13,14] Although the number of older patients is still too low to achieve statistical significance in the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analysis, the effect appears increasingly similar to that obtained in younger postmenopausal women. The role of anthracyclines in metastatic disease is less well defined, but they are effective. In a trial in patients who had not previously received anthracyclines, weekly epirubicin (Ellence) provided better survival than gemcitabine (Gemzar) in women aged 60 years and older.[15]
The best schedules appear to be low-dose deliveries, such as weekly epirubicin[16] or liposomal doxorubicin (Doxil).[17] The hazard ratio for cumulative cardiac toxicity (ie, congestive heart failure [CHF]) is 3.28 for patients 65 and older receiving 400 mg/m2 or more of doxorubicin, compared to younger patients.[18] Therefore, given the vast choice of alternative chemotherapies, we advise avoiding the use of anthracyclines for metastatic breast cancer in women who have already received anthracyclines as adjuvant treatment. If these agents do become necessary, one should give a regimen with low cardiac toxicity, and consider the addition of dexrazoxane.
Taxanes
Weekly paclitaxel administered at a dose of 80 mg/m2 on days 1, 8, and 15 has been assessed in two studies focusing on patients 70 and older.[19,20] Both found the drug to be effective. In the Dutch study, the primary reason for discontinuation was fatigue. In the Italian study, the occurrence of five cardiovascular events was noted in 41 patients. The clearance of unbound paclitaxel decreases with age, whether on a weekly or an every-3-week schedule.[21,22] This translates into an increased incidence of grade 3 or 4 neutropenia (with no major infectious consequences, however).[22]
Docetaxel every 3 weeks provides better survival than paclitaxel every 3 weeks in metastatic breast cancer patients.[23] Weekly paclitaxel is superior to every-3-week paclitaxel, but no direct comparison has been made between the two regimens in metastatic breast cancer. Weekly paclitaxel is associated with a higher neurotoxicity than every-3-week paclitaxel, and docetaxel weekly is complicated by skin and fatigue problems. All of these effects are items of concern for the elderly.
An attractive feature of docetaxel is its very simple pharmacokinetics. It is entirely metabolized by the cytochrome P450 subenzyme CYP3A4. Several methods exist to assess the activity of this enzyme; that activity highly correlates with the area under the concentration-time curve (AUC) of docetaxel, which, in turn, has some correlation with its toxicity.[24-28] There might be a potential for pharmacokinetics-based dose adaptation and a more reliable effectiveness/toxicity ratio in the elderly. Elderly patients, on average, take two drugs that interact with the CYP3A4 enzyme, and as such, a personalized functional assessment is useful in this setting.[29]
Capecitabine
The availability of capecitabine as an oral chemotherapy is attractive to many patients and physicians. Its safety in the elderly was evaluated recently in a phase II study.[30] After 2 deaths in the first 30 patients treated with 1,250 mg/m2 twice a day, the dose was reduced to 1,000 mg/m2 and well tolerated. These results are in line with the findings of the Cancer and Leukemia Group B (CALGB) 49907 study, which compared adjuvant capecitabine with standard AC (doxorubicin [Adriamycin], cyclophosphamide) or CMF (cyclophosphamide, methotrexate, fluorouracil) in women 65 and older, where an optional dose increase to 1,250 mg/m2 was eliminated after the occurrence of two deaths in that group. Therefore, capecitabine should not be given at doses above 1,000 mg/m2 to older women.
A Swiss Group of Clinical Cancer Research (SAKK) phase I study combined capecitabine and vinorelbine for women 65 and older with metastatic breast cancer.[31] The maximum -tolerated dose was vinorelbine,
20 mg/m2 on days 1 and 8, and capecitabine, 1,000 mg/m2/d (in two doses) on days 1 through 14 for women with bone involvement, and 1,250 mg/m2/d for women without bone involvement. This is a much lower dose than the one used in the capecitabine/docetaxel trial mentioned earlier. Caution should be exerted when combining capecitabine with another drug in the elderly woman.
A powerful way to decrease the side effects from capecitabine is to very systematically calculate creatinine clearance, even in women with a seemingly normal creatinine level. The latest dosage information for capecitabine requires dose reduction as soon as the creatinine clearance decreases below 45 mL/min. That said, with proper dose adjustment, capecitabine can be effective and tolerated even in very old patients, offering the option of a relatively nonhematotoxic treatment.
Gemcitabine
Gemcitabine is an easily tolerated regimen in the elderly. The dose-limiting toxicity is the hematologic toxicity. This often leads to a regimen of days 1 and 8 every 3 weeks rather than days 1, 8,and 15 every 4 weeks. The dose often has to be reduced from the baseline 1,000 mg/m2. This might be due, in part, to the fact that the half-life of gemcitabine is longer in the elderly and in women, although this has not been formally established by trial data.[32]
Targeted Therapies
Trastuzumab has been discussed briefly above. One analysis assessed specifically whether age was affecting outcome in the pivotal trastuzumab study.[33] The effect was actually better in older patients. The relative risk of mortality was 0.85 (0.66-1.09) for patients below age 60, and 0.64 (0.41-0.99) for those above age 60. Trastuzumab can induce CHF. No specific data regarding this effect are yet available in the elderly. It is important to remember, however, that this type of CHF is different from anthracycline-induced CHF: It easily responds to standard CHF therapy and is reversible.[34]
Another targeted therapy is bevacizumab (Avastin), an anti-VEGF monoclonal antibody. Two phase III studies of the drug in this setting are available. The first one compared capecitabine therapy with and without bevacizumab. The investigators found slightly more responses with the addition of bevacizumab but no difference in progression-free and overall survival.[35] The other, presented at the 2005 San Antonio Breast Conference, compared paclitaxel with or without bevacizumab and revealed a marked difference in progression-free survival and a difference in overall survival at very short follow-up.[36]
As far as the older woman is concerned, these results call for a few comments. First, it appears that the chemotherapeutic agent might not be a neutral substrate for the antibody. Paclitaxel has well known antiangiogenic properties, and there may be a synergistic effect between the two drugs. Second, these studies excluded patients with significant cardiovascular conditions. No data are thus far available to assess the safety of the use of bevacizumab in the large group of older patients with significant cardiovascular disease or diabetes. Risks and benefits should be carefully weighted for each patient.
Conclusions
Many options are available for the treatment of older patients with metastatic breast cancer. Hormonal therapy can provide several years of control, notably in patients with soft-tissue or bone-only disease. Multiple studies demonstrate that chemotherapy is well tolerated. In most cases, I advise sequential single-agent therapy. Although individual studies suggest an advantage for some combinations or agents, these should be reproduced to clarify the best approach in these patients.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Andre F, Slimane K, Bachelot T, et al: Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol 22:3302-3308, 2004.
2. McCloskey E: Effects of third-generation aromatase inhibitors on bone. Eur J Cancer 42:1044-1051, 2006.
3. Jenkins V, Shilling V, Fallowfield L, et al: Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology 13:61-66, 2004.
4. O'Shaughnessy J, Miles D, Vukelja S, et al: Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: Phase III trial results. J Clin Oncol 20:2812-2823, 2002.
5. Robert NJ, Leyland-Jones B, Asmar L, et al: Randomized phase III study of trastuzumab, paclitaxel, and carboplatin versus trastuzumab and paclitaxel in women with HER-2 overexpressing metastatic breast cancer: An update including survival (abstract 573). Proc Am Soc Clin Oncol 23:20, 2004.
6. Presant CA, Thompson E, Leberthon B, et al: Effects of weekly paclitaxel or paclitaxel plus carboplatin on functionality and symptoms of geriatric patients with cancer as measured by a brief geriatric oncology module: A pilot experience. Cancer 103:2623-2628, 2005.
7. Nabholtz JM, Senn HJ, Bezwoda WR, et al: Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol 17:1413-1424, 1999.
8. Extermann M, Chen H, Cantor AB, et al: Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur J Cancer 38:1466-1473, 2002.
9. Chen H, Cantor A, Meyer J, et al: Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer 97:1107-1114, 2003.
10. Eberhardt B, Dilger S, Musial F, et al: Short-term monitoring of cognitive functions before and during the first course of treatment. J Cancer Res Clin Oncol 132:234-240, 2006.
11. Eberhardt B, Dilger S, Musial F, et al: Medium-term effects of chemotherapy in older cancer patients. Support Care Cancer 14:216-222, 2006.
12. Hurria A, Rosen C, Zuckerman E, et al: Effect of adjuvant chemotherapy (CRx) on the cognitive function of older patients (pts) with breast cancer (BC): Results from a prospective study (abstract 8204). J Clin Oncol 23(16S):779s, 2005.
13. Early Breast Cancer Trialists' Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005.
14. Fargeot P, Bonneterre J, Roche H, et al: Disease-free survival advantage of weekly epirubicin plus tamoxifen versus tamoxifen alone as adjuvant treatment of operable, node-positive, elderly breast cancer patients: 6-year follow-up results of the French adjuvant study group 08 trial. J Clin Oncol 22:4622-4630, 2004.
15. Feher O, Vodvarka P, Jassem J, et al: First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: A multicenter, randomized, phase III study. Ann Oncol 16:899-908, 2005.
16. A prospective randomized trial comparing epirubicin monochemotherapy to two fluorouracil, cyclophosphamide, and epirubicin regimens differing in epirubicin dose in advanced breast cancer patients. The French Epirubicin Study Group. J Clin Oncol 9:305-312, 1991.
17. O'Brien ME, Wigler N, Inbar M, et al: Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15:440-449, 2004.
18. Swain SM, Whaley FS, Ewer MS: Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 97:2869-2879, 2003.
19. Del Mastro L, Perrone F, Repetto L, et al: Weekly paclitaxel as first-line chemotherapy in elderly advanced breast cancer patients: A phase II study of the Gruppo Italiano di Oncologia Geriatrica (GIOGer). Ann Oncol 16:253-258, 2005.
20. ten Tije AJ, Smorenburg CH, Seynaeve C, et al: Weekly paclitaxel as first-line chemotherapy for elderly patients with metastatic breast cancer. A multicentre phase II trial. Eur J Cancer 40:352-357, 2004.
21. Smorenburg CH, ten Tije AJ, Verweij J, et al: Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur J Cancer 39:196-202, 2003.
22. Lichtman SM, Hollis D, Miller AA, et al: Prospective evaluation of the relationship of patient age and paclitaxel clinical pharmacology: Cancer and Leukemia Group B (CALGB 9762). J Clin Oncol 24:1846-1851, 2006.
23. Jones SE, Erban J, Overmoyer B, et al: Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542-5551, 2005.
24. Yamamoto N, Tamura T, Kamiya Y, et al: Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol 18:2301-2308, 2000.
25. Yamamoto N, Tamura T, Murakami H, et al: Randomized pharmacokinetic and pharmacodynamic study of docetaxel: Dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol 23:1061-1069, 2005.
26. Extermann M, Hutson P, Gaffar Y, et al: Pharmacokinetics of weekly docetaxel in elderly patients: How well can it be predicted? Presented at the 5th Meeting of the International Society of Geriatric Oncology, San Francisco, October 15-16, 2004.
27. Fleming M, Baker S, Kelly WK, et al: Pharmacokinetics (pK) of weekly docetaxel in older patients with metastatic cancer. Presented at the 6th Meeting of the International Society of Geriatric Oncology, Geneva, 2005.
28. Bruno R, Vivier N, Veyrat-Follet C, et al: Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Invest New Drugs 19:163-169, 2001.
29. Extermann M, Overcash J, Wallace K, et al: Influence of concomitant medications metabolized by cytochrome P-450 on toxicity from chemotherapy. Presented at the International Conference on Geriatric Oncology. Rome, 2003.
30. Bajetta E, Procopio G, Celio L, et al: Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol 23:2155-2161, 2005.
31. Hess D, Thurlimann B, Pagani O, et al: Capecitabine and vinorelbine in elderly patients (> or =65 years) with metastatic breast cancer: A phase I trial (SAKK 25/99). Ann Oncol 15:1760-1765, 2004.
32. Lichtman SM, Skirvin JA, Vemulapalli S: Pharmacology of antineoplastic agents in older cancer patients. Crit Rev Oncol Hematol 46:101-114, 2003.
33. Fyfe GA, Mass R, Murphy M, et al: Survival benefit of trastuzumab (Herceptin) and chemotherapy in older (age>60) patients (abstract 189). Proc Am Soc Clin Oncol 20:48a, 2001.
34. Ewer MS, Vooletich MT, Durand JB, et al: Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol 23:7820-7826, 2005.
35. Miller KD, Chap LI, Holmes FA, et al: Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792-799, 2005.
36. Miller KD Wang M, Gralow J, et al: A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer: A trial coordinated by the Eastern Cooperative Oncology Group (E2100). Presented at the 28th San Antonio Breast Cancer Symposium, 2005.
Gedatolisib Combo With/Without Palbociclib May Be New SOC in PIK3CA Wild-Type Breast Cancer
December 21st 2025“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.