Update on Tyrosine Kinase Inhibitors (TKI) in Metastatic Renal Cell Carcinoma
A retrospective study of metastatic renal cell carcinoma (mRCC) patients published in the journal Cancer found that patients treated with tyrosine kinase inhibitors (TKIs) had better overall survival and less-frequent metastasis to the brain.
A retrospective study of metastatic renal cell carcinoma (mRCC) patients published in the journal Cancer found that patients treated with tyrosine kinase inhibitors (TKIs) had better overall survival and less-frequent metastasis to the brain (DOI: 10.1002/cncr.26138). Renal cell carcinoma comprises ~85% of all kidney cancers and is the 7th most commonly diagnosed cancer according to the American Cancer Society
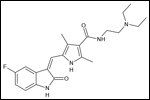
Structural diagram of sunitinib
The Aim and Methods
The study, conducted by researchers at the Baylor College of Medicine, aimed to evaluate the effect of TKIs on overall survival as well as brain metastasis in patients with metastatic
RCC. 338 patients with mRCC but no brain metastases were identified from institutional tumor registries and their outcomes were analyzed based on patient information, including type of treatment. 46% of patients with no brain metastases were treated with TKIs and 54% were not. The two groups had no difference in characteristics such as age, histology, and sites of metastases.
The Results
The authors found that the TKI-treated patient group had a median overall survival of 25 months compared with 12.1 months for the non-TKI group (P<0.0001, hazard ratio of 0.53). 13% of all patients developed brain metastases: 15.8% within the non-TKI group and 9.7% in the TKI-treated group. The study also found that metastasis to the lungs, the most frequent site of metastasis for RCC, increased the risk of metastasis to the brain.
Targeted Therapy Options for Advanced RCC
TKIs taken by patients in the study included sunitinib (Sutent) and sorafenib (Nexavar). Both are small-molecule anti-angiogenic drugs. Both TKIs target the vascular endothelial growth factor receptors (VEGFRs) and the platelet-derived growth factor receptor (PDGFR).
Another TKI, pazopanib (Votrient), was approved for advanced RCC by the FDA in October of 2009 for treatment-nave patients or those who have received previous cytokine therapy. Patients taking pazopanib were not part of this current study, as only patient registries up to 2007 were included in the analysis. Pazopanib is also a multi-target TKI that blocks VEGFRs and PDGRF as well as c-kit.
Novel TKIs in Development for RCC
RCC treatment is currently dominated by targeted treatments, including the three VEGFR inhibitors discussed above. An additional three agents are currently in phase 3 development: axitinib, tivozanib (AV-951), and TKI-258.
Axitinib, from Pfizer, is the most developmentally advanced. Pfizer announced in November 2010 that their phase 3 AXIS trial of previously treated metastatic RCC patients had met its primary endpoint of significantly extending progression free survival in RCC patients as compared to a standard treatment, sorafenib.
The actual study numbers and degree of benefit from axitinib will be presented at the American Society of Clinical Oncology (ASCO) Annual meeting in Chicago (AXIS trial, Abstracts 4503 and 4504, Monday June 6, 8:00am). Pfizer’s sunitinib is the dominant treatment-nave RCC patient treatment and most patients in the AXIS study have likely received this TKI as a first-line treatment. The analysis of the benefit with axitinib in patients grouped by prior treatment received will be interesting to note.
Tivozanib is being studied in a phase 3 trial (TIVO-1) that is scheduled to be completed by the end of 2011. The trial is also comparing the experimental agent to the standard of care, sorafenib, but in advanced RCC patients who have had at most one previous systemic treatment for RCC.
The third agent, TKI-258, is the least advanced with its phase 3 scheduled for completion in 2013. The trial is also comparing the novel TKI with sorafenib and is accruing patients who have had one previous VEFR-targeted and mammalian target of rapamycin (mTOR)-targeted therapy.