Ipsilateral regional nodal status is an important independent prognostic factor for patients with breast cancer. Several decisions regarding local therapy are necessary for patients found to have pathologically involved lymph node(s). This article reviews the role of completion dissection and/or radiation therapy in patients found to have positive sentinel lymph node(s), taking into consideration use of mastectomy vs lumpectomy, tumor characteristics, tumor biology, plans for systemic therapy, and patient preferences. Published literature and current guidelines are reviewed, with emphasis on controversial topics such as regional nodal and postmastectomy radiation therapy for patients with one to three positive nodes. Choice of field design in patients undergoing radiation therapy will also be highlighted. Unique clinical situations such as locoregional treatment in patients receiving neoadjuvant chemotherapy and the emerging role of tumor biology and molecular assays in local therapy decision making will also be discussed.
Introduction
Among patients with breast cancer, ipsilateral regional nodal status carries strong and independent prognostic value. The 5-year survival rates can range from less than 65% for patients with more than four involved axillary lymph nodes to greater than 95% for breast cancer patients with pathologically negative nodal disease.[1] This article will discuss the ways in which pathologic regional nodal status affects clinical decisions about locoregional and systemic treatment. In addition to reviewing published literature and guidelines, this review aims to provide a practical perspective on treatment approach in the face of a broad range of data and recommendations. Specific clinical questions, such as the need for locoregional treatment based on degree of nodal response to neoadjuvant chemotherapy, will be addressed in light of available data. Recent developments in gene expression assays and an increasing understanding of tumor biology may prove to be the next steps in answering these questions; the potential role of these emerging techniques will also be examined.
Staging of Axillary Lymph Nodes
Axillary lymph node dissection (ALND) with removal of level I–II axillary nodes was previously standard surgical management for early breast cancer. Over the last 2 decades, well-designed clinical trials have established that removal of sentinel lymph nodes (SLNs) and thorough pathologic assessment can be performed for patients with clinically node-negative disease and are associated with less morbidity, without compromising outcomes.[2] Sentinel lymph node biopsy (SLNB) is now the standard axillary procedure for T1-T2 clinically node-negative disease.[3,4] The role of SLNB in patients with large, locally advanced node-negative breast cancer (tumor size T3-T4) is not yet clear; in general, ALND is recommended both for these patients and for those with clinically involved axillary nodes.
Two main downstream decisions are required for local therapy in clinically node-negative patients who are found to have pathologically involved SLN(s). The first involves evaluation of the need for additional axillary treatment to establish local control (none vs ALND vs axillary radiation), and the second concerns the need for radiation therapy, including possible treatment to regional nodes and the chest wall. These decisions are sometimes intricately intertwined. In the subsequent sections, we will review the published literature and the clinical nuances that guide these two important treatment decisions.
Isolated Tumor Cells in Axillary Lymph Nodes
Isolated tumor cells are clusters of tumor cells no greater than 0.2 mm, or 200 cells, occurring in regional lymph node(s). They are detected by hematoxylin and eosin stain or immunohistochemistry. According to the TNM staging system, isolated tumor cell clusters are designated as pN0(i+). Included in this category are positive molecular findings (by reverse transcription polymerase chain reaction) in the absence of regional lymph node metastases detected by histology or immunohistochemistry, classified as pN0(mol+). Prognostically, patients with isolated tumor cell clusters in axillary lymph nodes appear to do as well as those with N0 disease, and decisions regarding systemic therapy, surgery, and radiotherapy for these patients should match those for node-negative patients.[5-7]
Using Lymph Node Pathology for Surgical Axillary Treatment Decisions
ALND omission in the setting of breast-conserving therapy and positive (N1mi/N1) SLNB
Historically, patients with any nodal metastasis noted on SLNB underwent completion ALND; however, fewer than 40% of patients were found to have additional pathologically involved axillary lymph nodes on ALND.[8,9] Two recent randomized trials have demonstrated that omission of ALND in many patients with node-positive breast cancer treated with breast conservation surgery (BCS) and adjuvant whole-breast radiation therapy (WBRT) does not compromise oncologic outcomes.[10-13]
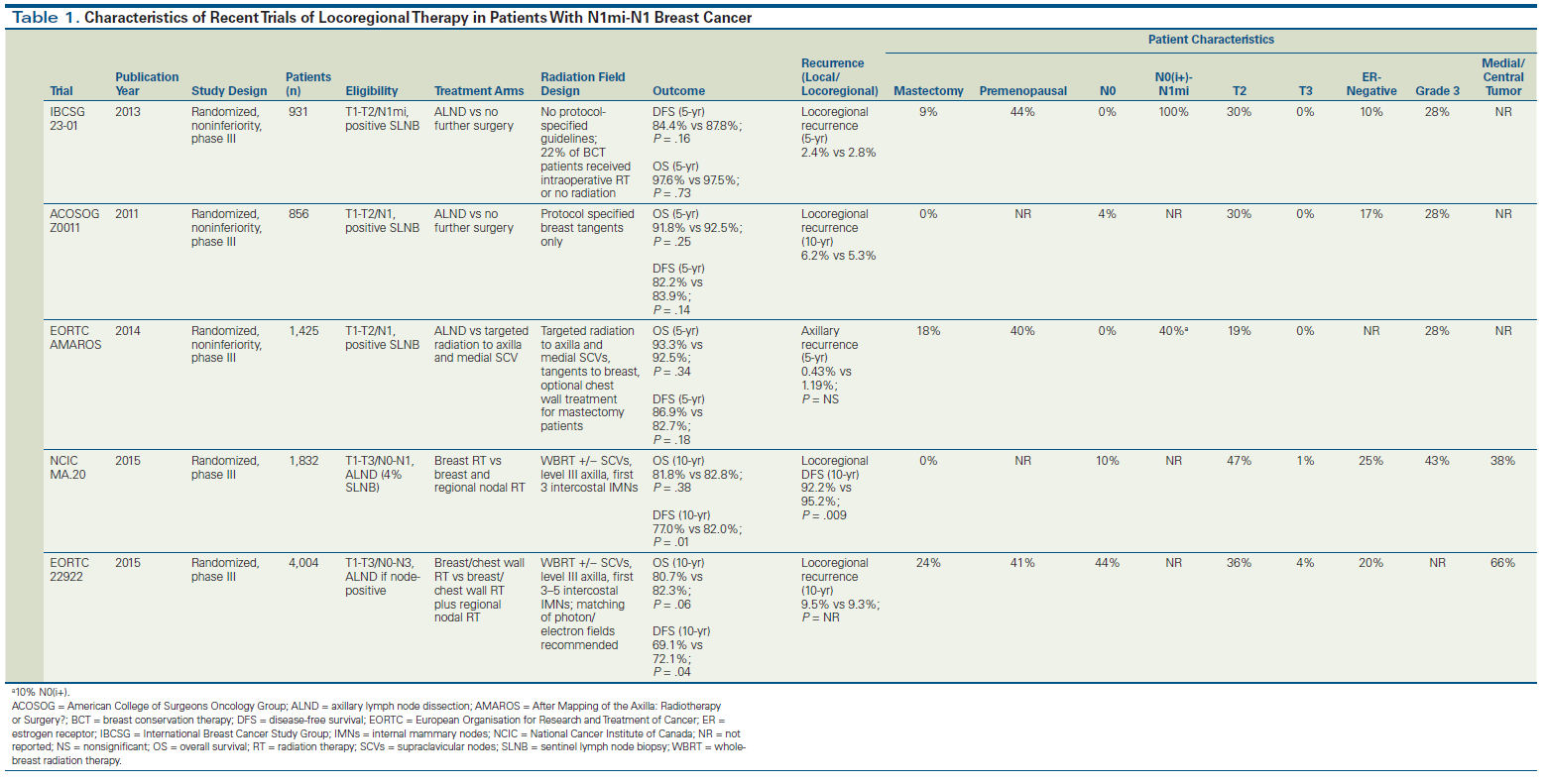
In the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, 891 patients with T1-T2 clinically node-negative breast cancer and one or two positive sentinel nodes, who were undergoing BCS followed by WBRT, were randomized to completion ALND after SLNB or SLNB alone. This study demonstrated similar 5-year overall survival (OS) and disease-free survival (DFS) in the ALND and SLNB-alone arms, with no difference in 10-year cumulative incidence of locoregional recurrences between the two arms (6.2% for ALND, 5.3% for SLNB alone; P = .36).[12] These results may not be surprising in light of the findings of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 study, which showed that patients without lymph node dissection, who had a predicted risk of harboring axillary disease of about 40%, developed local recurrences in that region only 20% of the time.[14] Furthermore, incidental axillary irradiation may also have helped to reduce the risk of recurrence, as will be discussed in further detail.
The International Breast Cancer Study Group (IBCSG) 23-01 trial randomly assigned 931 patients with T1-T2 clinically node-negative breast cancer and micrometastases seen on SLNB (N1mi) to either completion ALND or no further surgical intervention (SLNB alone). Although this study allowed inclusion of patients undergoing mastectomy, 91% of patients underwent BCS and received WBRT. Similar to the Z0011 study, the IBCSG study found no significant difference in DFS and OS between the ALND and SLNB-alone groups.[11]
Another study, the European Organisation for Research and Treatment of Cancer (EORTC) After Mapping of the Axilla: Radiotherapy or Surgery? (AMAROS) trial, also randomized T1-T2 clinically node-negative patients with a positive SLNB to completion dissection vs no further surgery.[10] Unlike the Z0011 study, in AMAROS the radiation fields were required to include draining lymph nodes of the undissected axilla, as well as the supraclavicular region. Similar to the results of the Z0011 trial, aggressive surgery conferred no benefits in terms of local control, DFS, or OS.
Despite some limitations (low accrual and a low event rate, underrepresentation of certain high-risk patients), the Z0011, AMAROS, and IBCSG trials have been practice changing. The current standard of care has now evolved to omission of ALND for many patients with low-volume axillary nodal disease who undergo BCS. The 2016 American Society of Clinical Oncology (ASCO) practice guidelines state that ALND should not be recommended for women with T1-T2 (≤ 5 cm) breast cancer who have one or two SLN metastases and will receive BCS with conventionally fractionated WBRT.[3] Omission of ALND should be applied cautiously in patients with tumors larger than 5 cm or gross extranodal extension, since these patient groups were not included in the aforementioned clinical trials.
ALND omission in the setting of mastectomy and positive (N1mi/N1) SLNB
Most patients with a positive SLNB who do not have absolute indications for radiotherapy should undergo ALND. Further prospective trials are needed to validate whether node-positive patients undergoing mastectomy without radiation are candidates for SLNB alone. The Z0011 trial excluded patients undergoing mastectomy and is therefore not applicable. Although IBCSG 23-01 enrolled mastectomy patients, this group represented only 9% of the trial population; furthermore, only N1mi patients were included. A retrospective study from Memorial Sloan Kettering Cancer Center (MSKCC) reviewed outcomes of SLNB-positive mastectomy patients who underwent SLNB without completion ALND or radiation. Most (54%) of the mastectomy patients in this study had N0(i+) disease, 37% had N1mi disease, and only 9% had N1 macrometastatic disease. Patients included in this study had low risk of additional axillary nodal involvement, with a median risk of nonsentinel axillary nodal metastasis of 9% per the MSKCC nomogram. Overall, the 4-year local and regional recurrence rates in this study were very low among mastectomy patients (1.7% and 1.4%, respectively) and remained low after N0(i+) patients were excluded from the analysis (1.2% and 2.5%, respectively).[15] While results are promising, completion ALND for mastectomy patients with a positive SLNB remains the standard of care, with the exception of situations where there are indications for postmastectomy radiation therapy (PMRT) based on existing pathology (see section in this article on PMRT decisions).
Absolute indications for completion ALND in patients with positive SLNB
Completion ALND is generally recommended for patients with T3-T4 disease and/or three or more positive SLNs, as well as patients who have one or two positive SLNs but will not undergo WBRT.
Additional high-risk features impacting axillary surgery
The presence of extranodal extension is associated with increased axillary nodal disease burden. The ACOSOG Z0011 trial (which excluded patients with gross extranodal extension) reported nonsentinel nodal metastases in 27% of patients; in contrast, the reported incidence of nonsentinel nodal metastases in patients with extranodal extension ranges from 58% to 84%.[12,16,17] Thus, omission of ALND should be considered cautiously in patients with sentinel node gross extranodal extension.[3]
Not all high-risk features have been conclusively addressed in clinical trials evaluating de-escalation of axillary surgery. Within the subset of T1-T2/N1mi-N1 breast cancers, additional high-risk factors beyond extranodal extension-such as young age, negative hormone receptor status, high histologic grade, lymphovascular space invasion, and size of nodal metastatic deposit-may be indicators of nonsentinel node involvement.[18-20] Most of these features were underrepresented or not reported in trials evaluating the efficacy of SLNB alone. The decision to omit ALND in T1-T2/N1mi-N1 disease when one or more of these high-risk features are present should be individualized based on the overall likelihood of both nonsentinel node involvement and the need for regional nodal irradiation (RNI).
Using Lymph Node Pathology in Locoregional Radiation Therapy Decisions
Several trials have demonstrated that addition of RNI to whole breast (with lumpectomy) or chest wall fields (with mastectomy) in patients with node-positive disease reduces locoregional recurrence.[21-26] Although reduction in locoregional recurrence was noted in all trials, improvement in OS and breast cancer–specific survival rates was not noted uniformly across trials. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) 2005 analysis demonstrated a quantitative relationship between local disease control and 15-year breast cancer mortality (namely, for every four local recurrences avoided, one breast cancer death over the next 15 years was avoided).[27] This analysis also demonstrated that there was little difference in 15-year breast cancer mortality in situations where local treatment comparisons produced < 10% difference in 5-year local recurrence risk. With the advancement of adjuvant therapy, the absolute risks of local recurrence in trials included in the EBCTCG analysis may not be generalizable to patients in the modern era; however, the quantitative relationships noted in this overview may be helpful in some situations to guide clinical decisions.
High axillary nodal disease burden (≥ N2 disease)
The absolute benefit (improvement in the rates of locoregional recurrence and OS) from radiotherapy in patients with node-positive disease is dependent on the baseline risk of locoregional recurrence, which in turn is influenced to a large degree by the number of involved axillary nodes.[27,28] Meta-analyses of intergroup trials and EBCTCG review have shown that presence of four or more positive axillary lymph nodes (ie, N2 and N3 disease) is associated with significantly increased risk of locoregional recurrence.[28] Thus, there is relatively little controversy over the management of N2 or N3 disease, and the American College of Radiology guidelines recommend RNI for these patients.[29] Recommendations regarding RNI for patients with N1 disease are a bit more complex and are discussed in further detail in the following section.
Intermediate axillary nodal disease burden (T1-T2/N1 disease)
Substitution of axillary radiation for ALND. While the ACOSOG Z0011 trial did not intend to evaluate nodal irradiation as an alternative to ALND, all patients received tangent radiation to the whole breast. It is possible that the favorable outcomes observed in the non-ALND arm of Z0011 were partly driven by unintentional axillary radiation. The average patient’s axilla lies anatomically partially within the tangential radiation field that covers the breast. Quality assessment of treatment plans for Z0011 have demonstrated that radiation fields for more than 50% of patients in the trial were within 2 cm of the humeral head, which would cover a significant portion of the level I and II axilla in an average patient.[30] The AMAROS trial expanded on these findings by comparing ALND against axillary node irradiation in 1,425 patients with T1-T2 clinically node-negative breast cancer and a positive SLNB.[10] Unlike the Z0011 study, radiation fields were required to include draining lymph nodes of the undissected axilla as well as the supraclavicular region. Similar to Z0011, there was no benefit for ALND over axillary irradiation in terms of local control, DFS, or OS. The main benefit of omitting ALND was a significant reduction in the rates of clinical lymphedema at 5 years (11% for radiation vs 23% for axillary dissection). Patients in the AMAROS trial had somewhat higher-risk disease compared with patients in the Z0011 study, since additional positive nodes at ALND were detected in 33% of patients in the surgical arm of the AMAROS trial, compared with 27% of patients in Z0011. Further comparisons of the differences between these trials are summarized in Table 1.
In summary, the outcomes of the two studies indicate that ALND can be avoided in patients with one or two positive SLNs who meet the criteria outlined for enrollment in the Z0011 trial. Patients who have clear indications for PMRT based on their SLNB pathology can also proceed to radiation without ALND; for these patients, the radiation oncologist should intentionally include the level I and II axilla in the radiation field by contouring these levels as described in the sections below.
RNI
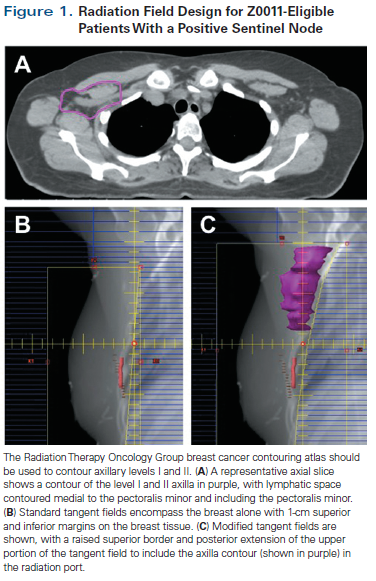
T1-T2/N1 patients undergoing breast-conserving therapy. Patients undergoing lumpectomy require adjuvant WBRT as a component of their breast conservation treatment. For Z0011-eligible lumpectomy patients with one or two positive SLNs who do not undergo ALND, the challenge lies in the design of the radiation field. In general, we support radiation to the level I and II axilla in all patients who omit ALND. This has minimal added morbidity for the average patient and does not preclude her from treatment with hypofractionated radiation. It is easy to include the entire axilla in the radiation field by paying careful attention to the radiation gantry angle and the design of the multileaf collimator blocks, as shown in Figure 1.
Decisions regarding the administration of radiotherapy to other regional nodes (supraclavicular, infraclavicular, internal mammary) in patients with T1-T2/N1 disease are more complex; for example, while the patient populations for Z0011 and AMAROS were very similar, patients on AMAROS received a third field of radiation directed at the high axilla and supraclavicular fossa.[10] Other trials targeting node-positive patients of similar risk evaluated more comprehensive radiation fields, including not only the supraclavicular fossa in the field, but also the internal mammary nodes.[24,26] The National Cancer Institute of Canada MA.20 trial randomly assigned lumpectomy-treated patients mainly with one to three positive nodes (although 10% were high-risk node-negative and 5% had more than three positive nodes) to either WBRT or WBRT plus RNI. This trial demonstrated a significant improvement with addition of RNI in 10-year locoregional recurrence–free survival (hazard ratio [HR], 0.59; P = .009; absolute improvement of 3%), DFS (HR, 0.76; P = .01; absolute improvement of 5%), and distant DFS (HR, 0.76; P = .03; absolute improvement of 4%), but not in OS (81.8% vs 82.8%; P = .38).[26] The EORTC 22922 trial included lumpectomy and mastectomy patients with primarily N0 or N1 disease and similarly showed significant improvement in DFS (HR, 0.89; P = .04; absolute improvement of 3%), distant DFS (HR, 0.86; P = .02; absolute improvement of 3%), and breast cancer–specific mortality (HR, 0.82; P = .02; absolute improvement of 1.9%) with addition of RNI to radiation to the breast/chest wall. Similar to the results of the MA.20 trial, the EORTC 22922 trial showed no improvement in OS.[24] Table 1 details the differences across these four studies with respect to patient and tumor characteristics.
Patient and tumor characteristics in the aforementioned trials have significant overlap, thus it can be difficult to make decisions regarding WBRT alone vs radiation to the breast and regional nodes in patients with one to three positive nodes. It is becoming increasingly important to identify subsets of patients for whom rates of locoregional recurrence are sufficiently low so as to permit the omission of RNI. In a prespecified subgroup analysis from MA.20, a greater treatment effect for RNI was noted in patients with estrogen receptor (ER)-negative disease, compared with patients with ER-positive disease. For patients with ER-negative disease, the 10-year OS rate was 73.9% in the control group, compared with 81.3% in the RNI group, a difference that approached statistical significance (HR, 0.69; P = .05). Improvement in DFS with the addition of RNI was also more robust for patients with ER-negative disease (HR, 0.56) compared with those with ER-positive disease (HR, 0.88).[26] These findings suggest that hormone receptor status should be strongly weighed in the decision to treat with comprehensive nodal radiation.
Furthermore, the results of several recent studies support the association between hormone receptor status and rates of locoregional recurrence. A recent analysis from NSABP B-28 demonstrated that lumpectomy patients with hormone receptor–positive, node-positive breast cancer who receive WBRT and contemporary adjuvant chemotherapy plus endocrine therapy have a low 11-year rate of locoregional recurrence (6.5%).[31] Similar observations for hormone receptor–positive disease have been reported by others.[32-34] Molecular diagnostics can help us further refine the criteria for selection of appropriate candidates for RNI. Gene expression assays like the Oncotype DX 21-gene recurrence score and others predict risk of breast cancer recurrence and aid in systemic therapy decisions for patients with hormone receptor–positive disease. Beyond assessment of clinical and pathologic factors, molecular tools are rapidly evolving to help identify patients at high risk of locoregional recurrence. Recent data suggest that gene expression assays can independently predict locoregional recurrence.
Investigators from the NSABP B-28 trial recently reported locoregional recurrence to be associated with the 21-gene recurrence score in ER-positive, node-positive patients treated with adjuvant chemotherapy plus tamoxifen. Patients on NSABP B-28 were treated with WBRT, but RNI and PMRT to the chest wall were prohibited.[31] In univariate analyses, recurrence score was a statistically significant predictor of locoregional recurrence (with 10-year cumulative incidence rates at 3.3%, 7.2%, and 12.2% for low, intermediate, and high recurrence scores, respectively; P < .001). In multivariable regression analysis, recurrence score remained an independent predictor of locoregional recurrence, along with pathologic nodal status and tumor size.
If appropriately validated in other studies, the 21-gene recurrence score may aid in identifying patients with higher risk of locoregional recurrence who would thus benefit from RNI. Prospective studies are needed to further clarify the role of gene expression assays in clinical decision making with respect to postlumpectomy RNI and postmastectomy chest wall radiation and RNI in patients with hormone receptor–positive, node-positive breast cancer.
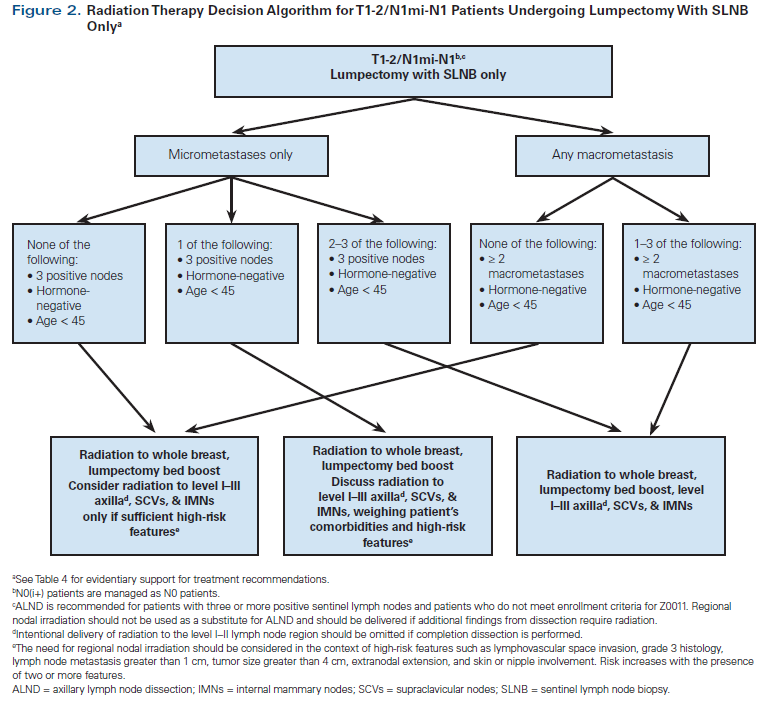
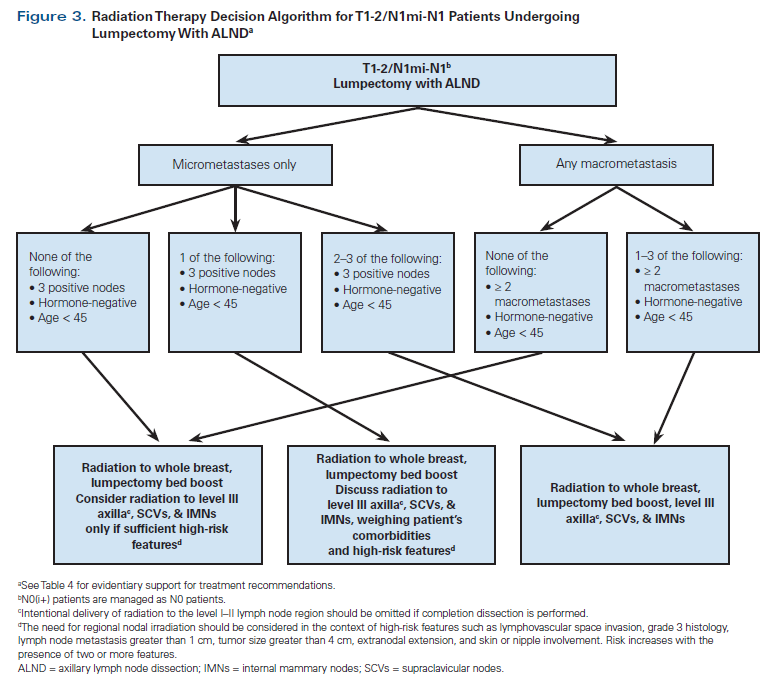
Additional clinical/pathologic factors beyond hormone receptor status, such as age, histologic grade, lymphovascular invasion, extranodal extension, size of node metastasis, and tumor size, have been reported to be associated with risk of locoregional recurrence and should be considered in RNI decision making for individual patients.[34-36] Patient participation in the choice of nodal radiation is encouraged, and thorough discussion of treatment risks and benefits is recommended. Referral to a basic algorithm can be a starting point for this conversation. Our institutional preferences for radiation to the breast and low axilla vs more comprehensive nodal treatment in patients undergoing BCS are outlined in Figures 2 and 3. Our algorithms are generally adapted from published guidelines.[4,36] However, based on the available literature and to streamline decision making and reduce practice variations across our institution, we have also included some high-risk features like negative hormone receptor status, younger age, and greater number of involved lymph nodes into our algorithm.
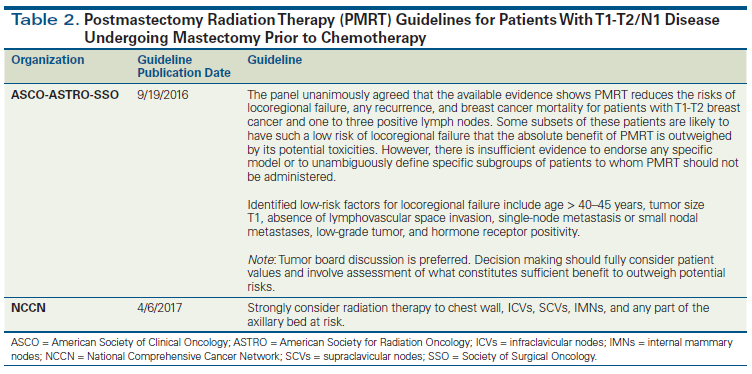
T1-T2/N1 mastectomy patients. Randomized trials and overview analyses have suggested that PMRT improves outcomes for patients with T1-T2 breast cancer and one to three positive lymph nodes; however, controversy exists regarding the applicability of these older studies to patients treated in the modern era (Table 2).[21-25] One of the main reasons for the continuing debate is that rates of locoregional recurrence in the control arm (ie, the arm in which patients did not receive radiation) in the historical PMRT trials and overview analyses are considerably higher (21%) than those reported in more recent adjuvant trials such as NSABP B-28 (7.2%).[21,31] Retrospective data also suggest that PMRT may not benefit patients with N1 disease treated in the modern era. A retrospective study reported locoregional recurrence rates in patients with T1-T2/N1 breast cancer treated with mastectomy and adjuvant chemotherapy with or without PMRT during an early era (1978–1997) and a later era (2000–2007). PMRT reduced the rate of locoregional recurrence in the early cohort (5-year rate of 9.5% without PMRT and 3.4% with PMRT; P = .028); however, it did not appear to benefit patients treated in the later cohort, with very low 5-year rates of 2.8% without PMRT and 4.2% with PMRT (P = .48).[37]
It is evident that overall breast cancer recurrence rates have decreased significantly for patients with N1 disease since the publication of results from trials of PMRT. This decrease is thought to be multifactorial; it results from both improvement in adjuvant systemic therapy (which impacts systemic recurrence rates as well as rates of locoregional recurrence) and from more sensitive clinical and pathologic nodal staging.
With low event rates noted for the average patient with N1 breast cancer in the modern era, there is unlikely to be a significant survival advantage from adding PMRT for unselected N1 patients. All N1 cases should be evaluated at regular multidisciplinary tumor conferences, and the risks and benefits of local therapy should be thoroughly discussed with all patients. Similar to optimizing decision making for patients undergoing BCS, PMRT decisions in mastectomy patients with one to three positive nodes require consideration of hormone receptor status, tumor biology, and high-risk factors. For patients who are deemed candidates for PMRT, comprehensive radiation to include the supraclavicular region and internal mammary nodes is recommended, based on trials that have shown that this approach yields a significant improvement in DFS.[24,26]
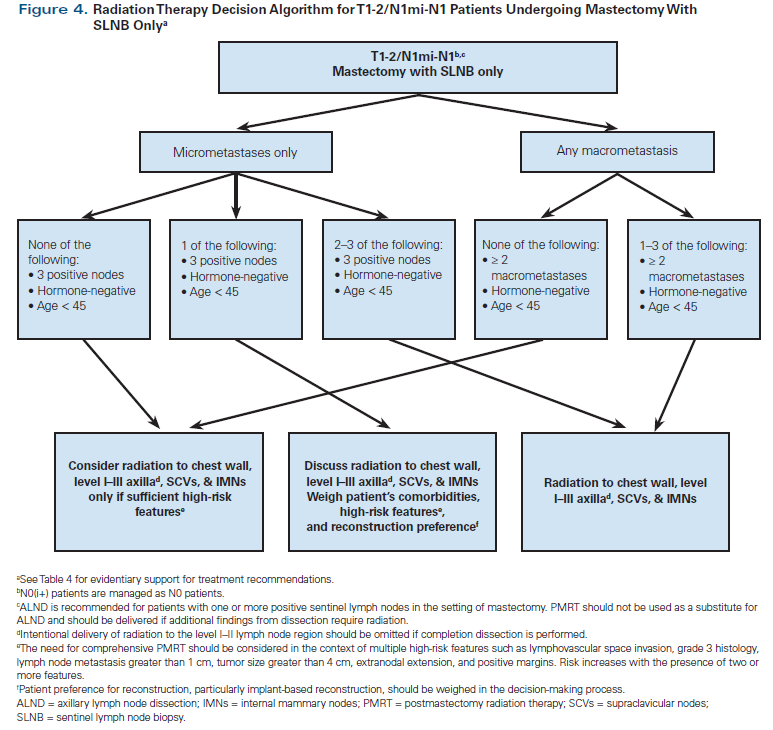
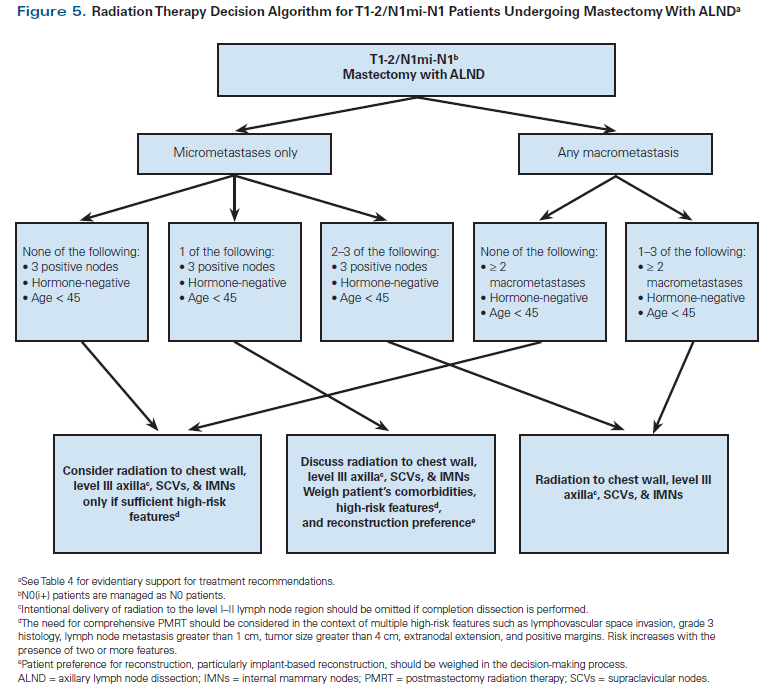
Our own institutional algorithm is shown in Figures 4 and 5. It is hoped that the ongoing SUPREMO (Selective Use of Postoperative Radiotherapy After Mastectomy) trial, which randomizes N1 patients to PMRT vs observation, will shed some light on appropriate patient selection for PMRT in the modern era.
The Complexities of Neoadjuvant Chemotherapy
Over the last decade there has been increasing use of preoperative or neoadjuvant chemotherapy in the management of breast cancer, especially for patients with hormone receptor–negative disease. Neoadjuvant chemotherapy has resulted in DFS and OS rates equivalent to those achieved with adjuvant chemotherapy, while yielding a 10% to 30% increase in eligibility for breast conservation treatment.[38-40] Response to neoadjuvant chemotherapy represents an additional indicator of prognosis, with pathologic complete response (pCR) in the primary tumor and lymph nodes being associated with significantly improved DFS and OS.[41-44] Furthermore, in the NSABP B-18 and B-27 trials, patients treated with neoadjuvant chemotherapy had a 30% to 40% reduction in axillary node positivity compared with those who received adjuvant chemotherapy.[38,45] The ability of neoadjuvant chemotherapy to reduce the extent of axillary disease adds another layer of complexity to local therapy decision making.
For patients with clinically node-negative disease, SLNB following neoadjuvant chemotherapy is considered an acceptable approach.[46] These recommendations are based on surgical outcomes data from NSABP B-27 and findings from a meta-analysis of 21 studies, both of which demonstrated that sentinel node identification and false-negative rates for SLNB after neoadjuvant chemotherapy are similar to those obtained with SLNB at the time of primary surgery.[45,47] Patients with clinically node-negative disease and negative SLNB after neoadjuvant chemotherapy do not require further axillary treatment.
Surgical axillary management for clinical N1 disease in the context of neoadjuvant chemotherapy
The success of SLNB after neoadjuvant chemotherapy in clinically node-negative disease, coupled with increasing rates of pCR in patients who receive modern chemotherapy regimens and targeted treatment, led to heightened interest in evaluating SLNB after neoadjuvant chemotherapy in clinical N1 disease.[48-50]
Several trials have tested the feasibility of SLNB after neoadjuvant chemotherapy in patients presenting with clinical N1 disease.[48-50] In the SENTINA trial, 592 patients with clinical N1 breast cancer treated with neoadjuvant chemotherapy underwent SLNB followed by ALND. The false-negative rate was 18.5% if two or more nodes were removed, suggesting that this should not be a routine procedure.[50] The Sentinel Node Biopsy Following Adjuvant Chemotherapy study mandated use of immunohistochemistry for detection of positive nodes and reported a lower false-negative rate of 13.3%.[48] In the ACOSOG Z1071 trial, the primary endpoint was false-negative rate with a limit of 10%, which was the rate expected for women with clinically node-negative disease undergoing SLNB.[49] The false-negative rate in the Z1071 study was 12.6% (95% CI, 9.9%–16.1%), which was higher than the preset limit, although the SLN detection rate of 92.9% was comparable to that of patients with clinical N0 disease.[45,47] A subset analysis showed that use of dual mapping agents and recovery of more than two SLNs were associated with lower false-negative rates of 10.8% and 9.1%, respectively.[49] For patients with clinical N1 disease with two or more SLNs removed, retrieval of a radiopaque clip within the SLNB specimen resulted in a false-negative rate of 6.8% (95% CI, 1.9%–16.5%). It is possible that ALND could be safely avoided in clinically N1 patients who become clinically N0; however, adequately powered prospective trials with follow-up survival data collected over a longer period of time are needed before this approach can become a standard of care.
Current guidelines from the National Comprehensive Cancer Network state that SLNB can be performed on select patients with clinical N1 breast cancer who have clinically negative axilla after neoadjuvant chemotherapy, and that the SLNB false-negative rate can be improved by removing more than two lymph nodes, using dual tracers, and marking biopsied lymph nodes to document their removal.[4] Consistent with these guidelines, our own institutional approach is to perform SLNB in patients with clinical N1 disease who have converted to clinical N0 status under certain conditions only; we limit this technique to patients who have had a biopsy clip placed in the node to mark the site at the time of diagnosis, who are evaluated with a dual tracer technique (usually blue dye and a particulate radioisotope), and in whom at least two lymph nodes are retrieved in the SLNB specimen. In patients with a negative SLNB under these guidelines, we do not recommend completion ALND. For patients with persistent positive nodal disease on SLNB, ALND is the standard of care.
KEY POINTS
- Clinical trials conducted in recent years have identified a subset of breast cancer patients with low-volume axillary nodal disease for whom completion nodal dissection can be safely omitted.
- For patients who receive neoadjuvant chemotherapy, extent of nodal response predicts risk of locoregional and systemic recurrence and can guide decisions regarding nodal irradiation.
- Ongoing clinical trials may clarify which subsets of patients with N1 disease benefit from axillary completion dissection and/or regional nodal irradiation.
It is also possible that among patients with N1 disease, the most appropriate candidates for SLNB after neoadjuvant chemotherapy are those with the highest degree of nodal response on clinical evaluation and imaging. An ongoing study (Alliance A011202) aims to decrease the extent of nodal surgery in patients with clinical nodal response to neoadjuvant chemotherapy. Patients participating in this study undergo SLNB, and those found to have pathologically involved SLNs are randomized between completion dissection or targeted radiation to the undissected axilla. All patients receive standard radiation to the whole breast/chest wall, internal mammary nodes, supraclavicular region, and high axilla. Results from A011202 are expected to clarify the role of a positive SLNB for patients with clinical N1 disease that persists at the time of surgery in the setting of neoadjuvant chemotherapy.
Radiation management of patients with clinical N1 disease who achieve nodal pCR with neoadjuvant chemotherapy
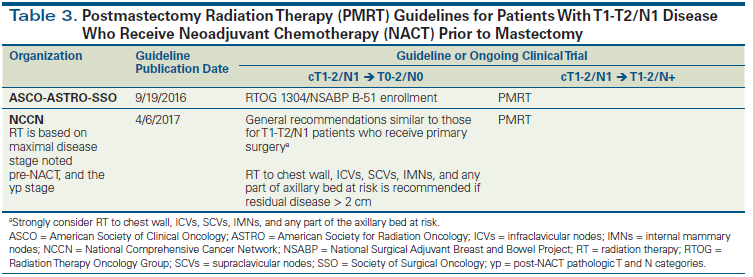
Achievement of pCR in breast tumors and lymph nodes of patients who receive neoadjuvant chemotherapy is an indicator of excellent long-term prognosis.[42,51] Patients undergoing BCS after neoadjuvant chemotherapy should all receive WBRT, regardless of the pathologic response to treatment. With the increasing use of neoadjuvant chemotherapy, a commonly encountered clinical scenario involves patients who present with pathologically involved axillary nodes, receive neoadjuvant chemotherapy, and are rendered pathologically node-negative at the time of definitive surgery. For such patients, there is an ongoing debate about the appropriate extent of locoregional radiation after mastectomy or BCS. One could argue that these patients present with pathologically involved axillary nodes (with uncertainty around the exact number of involved lymph nodes at presentation) and are at high risk for locoregional recurrence; therefore, they should receive radiation to the chest wall and RNI (after mastectomy) or WBRT plus RNI (after lumpectomy). However, neoadjuvant trials have demonstrated that, compared with pretreatment lymph node status, post–neoadjuvant chemotherapy lymph node status is a more robust indicator for DFS.[52] Furthermore, sterilization of involved axillary nodes by neoadjuvant chemotherapy lowers the risk for locoregional recurrence, making the potential benefit from chest wall irradiation and RNI (after mastectomy) and from RNI (after lumpectomy) uncertain. This uncertainty is also reflected in the range of recommendations noted in the published RNI guidelines for N1 patients who undergo neoadjuvant chemotherapy (Table 3). In the modern era, radiation therapy decisions are complicated further by the desire of most patients undergoing mastectomy to receive immediate reconstruction.
An ongoing randomized phase III trial, Radiation Therapy Oncology Group (RTOG) 1304/NSABP B-51, is evaluating the role of radiation therapy in patients with documented positive axillary nodes (T1-T3/N1) who convert to pathologically node-negative disease after neoadjuvant chemotherapy. The trial is designed to determine whether patients who undergo BCS can be treated with adjuvant breast radiation alone or if they require additional nodal radiation, and whether or not chest wall and axillary radiation improves postmastectomy outcomes, compared with no radiation therapy. We encourage patients who meet the eligibility criteria to enroll in this important study. For patients who have complete pathologic lymph node response but either do not have access to the trial or are not eligible to enroll, we recommend an individualized approach weighing in tumor biology, age, presenting tumor size, in-breast response, and reconstruction desires.[53,54] Our general bias in this situation is towards recommending RNI for young patients (< 45 years old), hormone receptor–negative patients, and for patients presenting with T3 disease, even in the setting of complete pathologic nodal response (regardless of pathologic response in the breast).
Radiation management of patients with clinical N1 disease with residual nodal disease after neoadjuvant chemotherapy
Combined analysis of the NSABP B-18 and B-27 trials of neoadjuvant chemotherapy in patients with operable breast cancer, neither of which allowed RNI or chest wall radiation, has demonstrated that residual nodal disease is associated with extremely high locoregional recurrence rates in the range of 15% to 22%.[53] Therefore, all patients with persistent nodal disease after neoadjuvant chemotherapy should be routinely treated with comprehensive RNI and chest wall radiation therapy (if undergoing mastectomy) or WBRT with comprehensive RNI (if undergoing lumpectomy)[4] (Table 3). Enrollment in the aforementioned Alliance A011202 trial is highly encouraged.
Locoregional management of clinical N2-N3 disease in the setting of neoadjuvant chemotherapy
Patients presenting with clinical N2-N3 disease should undergo ALND, since both the feasibility and false-negative rate of SLNB have not been studied in patients with N2-N3 disease. These patients have a high rate of locoregional recurrence regardless of the degree of pathologic response; thus, comprehensive RNI is recommended for these patients irrespective of response to neoadjuvant chemotherapy.[54]
The Interplay Between Decisions About Radiation Therapy, Surgery, and Breast Reconstruction
Cosmesis is intimately tied to quality of life in breast cancer survivors. PMRT to the chest wall and regional nodes can interfere with the success of implant-based reconstruction, often limiting a patient’s surgical options to more invasive procedures, such as deep inferior epigastric perforators or latissimus flap reconstruction. These procedures are associated with long anesthesia duration, extended recovery time, and often multiple-stage surgeries; therefore, not all patients are candidates for, or want to pursue, them.
For patients who are lumpectomy candidates and require RNI, we favor lumpectomy over mastectomy, since WBRT plus RNI is likely to provide superior cosmetic outcomes, with less morbidity and at a lower cost compared with RNI and PMRT to the chest wall. Patients expressing the desire to undergo mastectomy in the hope of avoiding radiation therapy should be counseled regarding the potential need for chest wall radiation and RNI and educated about the nuanced clinical decision making associated with breast reconstruction and PMRT.
Summary
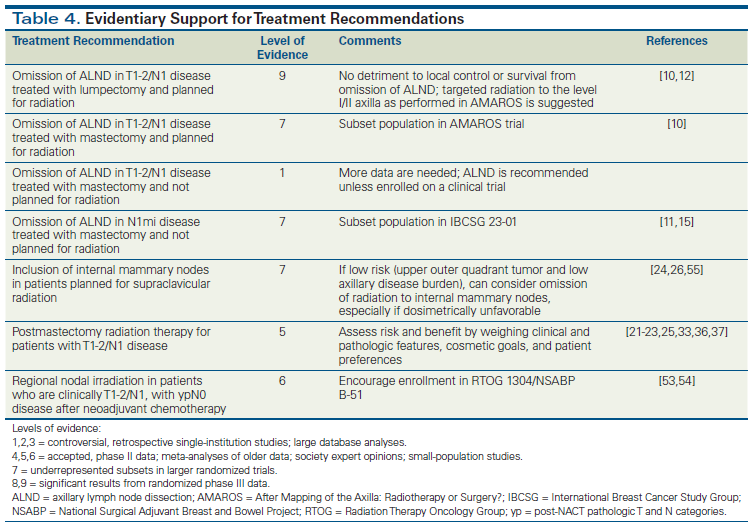
The past decade has witnessed rapid changes in local management of node-positive breast cancer, with the publication of new study data and society guidelines; however, many questions remain. Table 4 summarizes the quality of data associated with recommendations provided in this review. Current ongoing trials may clarify which subsets of patients with N1 disease benefit from axillary completion dissection and/or RNI.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181-7.
2. Krag DN, Julian TB, Harlow SP, et al. NSABP-32: phase III, randomized trial comparing axillary resection with sentinel lymph node dissection: a description of the trial. Ann Surg Oncol. 2004;11(3 suppl):208S-210S.
3. Lyman GH, Somerfield MR, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: 2016 American Society of Clinical Oncology clinical practice guideline update summary. J Oncol Pract. 2017;13:196-8.
4. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer. Version 4.2017; February 7, 2018. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed February 12, 2018.
5. Chagpar A, Middleton LP, Sahin AA, et al. Clinical outcome of patients with lymph node-negative breast carcinoma who have sentinel lymph node micrometastases detected by immunohistochemistry. Cancer. 2005;103:1581-6.
6. Reed J, Rosman M, Verbanac KM, et al. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center Sentinel Node Multicenter Study. J Am Coll Surg. 2009;208:333-40.
7. Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412-21.
8. Giuliano AE, Jones RC, Brennan M, et al. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345-50.
9. Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864-7.
10. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303-10.
11. Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297-305.
12. Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264:413-20.
13. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569-75.
14. Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567-75.
15. Milgrom S, Cody H, Tan L, et al. Characteristics and outcomes of sentinel node-positive breast cancer patients after total mastectomy without axillary-specific treatment. Ann Surg Oncol. 2012;19:3762-70.
16. Choi AH, Surrusco M, Rodriguez S, et al. Extranodal extension on sentinel lymph node dissection: why should we treat it differently? Am Surg. 2014;80:932-5.
17. Abdessalam SF, Zervos EE, Prasad M, et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg. 2001;182:316-20.
18. Kohrt HE, Olshen RA, Bermas HR, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66.
19. Mittendorf EA, Hunt KK, Boughey JC, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255:109-15.
20. Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140-51.
21. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127-35.
22. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b trial. N Engl J Med. 1997;337:949-55.
23. Overgaard M, Jensen M, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641-8.
24. Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317-27.
25. Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956-62.
26. Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307-16.
27. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-106.
28. Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247-54.
29. Taylor ME, Haffty BG, Rabinovitch R, et al. ACR appropriateness criteria on postmastectomy radiotherapy expert panel on radiation oncology-breast. Int J Radiat Oncol Biol Phys. 2009;73:997-1002.
30. Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) trial. J Clin Oncol. 2014;32:3600-6.
31. Mamounas EP, Liu Q, Paik S, et al. 21-gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017;109.
32. Fitzal F, Filipits M, Rudas M, et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial. Br J Cancer. 2015;112:1405-10.
33. Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419-26.
34. Tseng YD, Uno H, Hughes ME, et al. Biological subtype predicts risk of locoregional recurrence after mastectomy and impact of postmastectomy radiation in a large national database. Int J Radiat Oncol Biol Phys. 2015;93:622-30.
35. Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735-42.
36. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. J Clin Oncol. 2016;34:4431-42.
37. McBride A, Allen P, Woodward W, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys. 2014;89:392-8.
38. Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165-74.
39. Fisher ER, Costantino J, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) protocol B-17: intraductal carcinoma (ductal carcinoma in situ). Cancer. 1995;75:1310-9.
40. van Nes JG, Putter H, Julien JP, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up: clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009;115:101-13.
41. Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329-34.
42. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-72.
43. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275-81.
44. von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623-30.
45. Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694-702.
46. American Society of Breast Surgeons. Performance and practice guidelines for the use of neoadjuvant systemic therapy in the management of breast cancer. Approved March 2, 2017. https://www.breastsurgeons.org/statements/guidelines/PerformancePracticeGuidelines_NST.pdf. Accessed February 12, 2018.
47. Xing Y, Foy M, Cox DD, et al. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539-46.
48. Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258-64.
49. Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455-61.
50. Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609-18.
51. Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049-60.
52. Sikov W, Berry D, Perou C, et al. Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/− carboplatin and/or bevacizumab in triple-negative breast cancer: outcomes from CALGB 40603 (Alliance). Cancer Res. 2016;76(4 suppl):abstr S2-05.
53. Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960-6.
54. McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004-9.
55. Thorsen LB, Offersen BV, Dano H, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314-20.