USPSTF Says No Screening for Ovarian Cancer in New Guidelines
In updated guidelines, the USPSTF concludes that there is still no adequate evidence of a mortality benefit from routine ovarian cancer screening using transvaginal ultrasonography or CA-125 testing.
In updated guidelines, the US Preventive Services Task Force (USPSTF) concluded that there is still no adequate evidence of a mortality benefit from routine ovarian cancer screening using transvaginal ultrasonography or single-threshold serum CA-125 testing. The recommendations were published last week in the Annals of Internal Medicine.
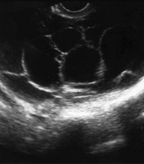
Ultrasound images of multiple septated cysts. Image courtesy of Edward J. Pavlik, director of research in gynecologic oncology, UK Ovarian Screening Research Program.
Most ovarian cancers are diagnosed at a late stage, resulting in the cancer being the fifth leading cause of cancer death among women. Many symptoms of the disease-such as abdominal bloating and pressure, persistent indigestion and gas, and low back pain-are common to other bladder and digestive disorders.
“Women with abdominal symptoms should include ovarian cancer in the differential of possible causes,” said Stephanie V. Blank, MD, an obstetrician/gynecologist and associate professor at the NYU Langone Medical Center in New York. “Women with suspected ovarian cancer should be seen by a gynecologic oncologist.”
Because ovarian cancer is infrequent in the general population, about 13 cases per 100,000 women (21,000 diagnoses per year), the value of ovarian cancer screening is not high.
The USPSTF recommendation against ovarian cancer screening reaffirms previous guidelines from the task force. That these tests can do more harm than good in part led to the consensus against screening. The panel concluded that there is at least moderate harm associated with ovarian cancer screening, resulting from the number of false positives that require extra tests and surgery.
“The updated guidelines do not differ from prior ones. They are a reaffirmation of previous statements,” said Dr. Blank. “The issues were revisited to incorporate newer data.”
The results from three trials were analyzed for the updated guidelines. The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that CA-125 testing and ultrasonography did not result in an improvement of either cancer-specific or overall mortality compared with standard care. According to the USPSTF, the PLCO trial also showed a potential harm from screening due to the frequency of false positives. Two other randomized, controlled trials-the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) and Shizuoka Cohort Study of Ovarian Cancer Screening (SCSOCS) showed similar results for potential harms, but did not have conclusions on mortality rates.
“There is no role for ovarian cancer screening in the general population,” said Dr. Blank, highlighting that false positives are an especially big issue when dealing with a relatively rare disease.