Zanubrutinib Appears to Elicit Promising Activity in Relapsed/Refractory MCL
Patients with relapsed/refractory mantle cell lymphoma experienced promising response rates after undergoing treatment with zanubrutinib.
Zanubrutinib (Brukinsa) yielded positive response rates and demonstrated tolerability in patients with relapsed/refractory mantle cell lymphoma (MCL), according to the results of a phase 1/2 study (NCT02343120) published in Blood Advances.1
Data from the trial indicated that patients achieved an overall response rate (ORR) of 84%, including a 25% complete response (CR) rate after undergoing treatment with zanubrutinib. Additionally, patients experienced a median duration of response (DOR) of 18.5 months and a median progression-free survival (PFS) of 21.1 months.
“The results presented here add to a now large and growing body of zanubrutinib data, demonstrating its benefits, including potent durable responses and an encouraging safety profile in patients with MCL who have received 1 [or more] prior [therapies],” the authors of the study wrote. “In addition, the results indicate that zanubrutinib can be administered once or twice daily, providing a flexible dosing schedule and potentially improving adherence to therapy and ensuring sustained responses.”
The first in human, multicenter, open label study examined the efficacy of zanubrutinib in patients with B-cell malignancies and enrolled patients from 24 sites across 6 countries. Phase 1 of the study utilized a dose escalation design to identify the recommended phase 2 dose and part 2 focused on disease-specific cohorts of patients with B-cell malignancies, including MCL, chronic lymphocytic leukemia/small lymphocytic lymphoma, and Waldenström macroglobulinemia. Although the maximum tolerated dose was not identified, the recommended phase 2 dose was identified as 160 mg twice daily or 320 mg daily.
Investigators enrolled 16 treatment-naïve patients and 37 patients with relapsed/refractory disease for part 1 and 2. A total of 6 patients with relapsed/refractory disease were enrolled in part 1 and given zanubrutinib at a dose of either 160 mg or less (n = 5) or 320 mg (n = 1). Thirty-one patients with relapsed/refractory MCL were treated with either 160 mg twice daily (n = 14) or 320 mg daily (n =17).
Eligible patients needed to be aged 18 years or older with an ECOG performance status of 0 to 2 and adequate hematologic, renal, and liver function. Previous exposure to a Bruton tyrosine kinase inhibitor was not allowed. Key exclusion criteria included central nervous system involvement, significant cardiac disease, and allogenic stem cell transplant within 6 months of enrolling on the study.
From September 22, 2014, to March 22, 2018, investigators enrolled 37 patients on the trial. Patients had a median of 1 prior therapy and a total of 30 patients had previously undergone treatment with rituximab or a rituximab-containing regimen.
The median time on study was 18.8 months, with patients undergoing treatment for a median of 15.4 months. At the time of data cutoff, 44% of patients continued to receive treatment with zanubrutinib while 56% had discontinued. The most common reasons for discontinuation were progressive disease (31.3%) and adverse effects (AEs; 25%).
Additional findings indicate that at 6 and 12 months, 83.3% and 78.7% of patients were still responding to treatment, respectively. A total of 43.8% of patients experienced progressive disease and died and 56.3% were censored. Investigators reported that 87.3% of patients were event free at 6 months, as well as 73.0% at 12 months, respectively. The 12- and 24-month OS rates were 83.0% and 64.4%, respectively.
Adverse effects of special interest associated with zanubrutinib in relapsed/refractory mantle cell lymphoma.
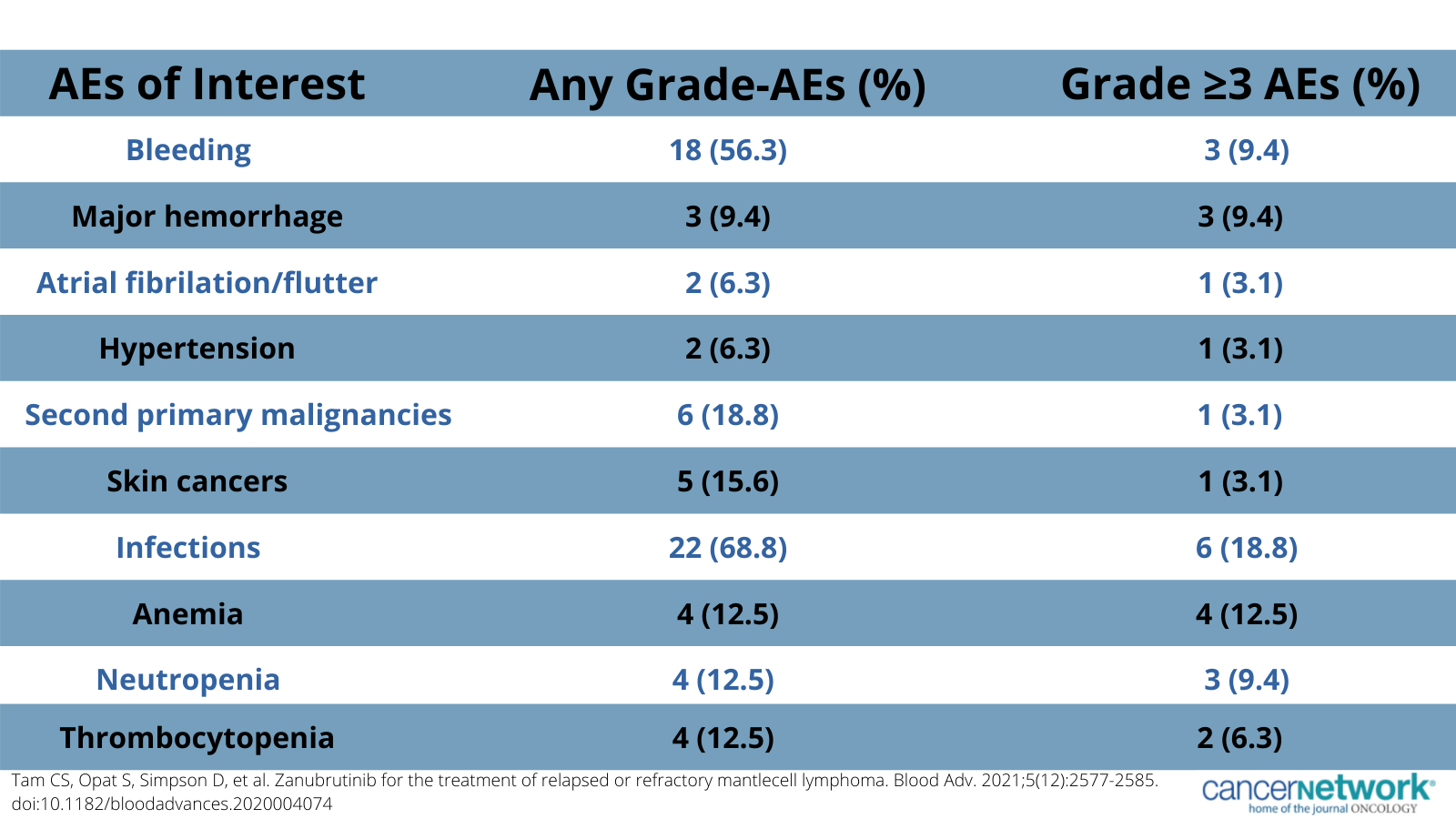
In terms of safety, 96.9% of patients reported 1 or more AEs of any grade and 59.4% reported 1 or more AEs of grade 3 or higher. Commonly reported any grade AEs included diarrhea (43.8%), contusion (37.5%), constipation (31.3%), and upper respiratory tract infection (31.3%). The most common grade 3 or higher AEs included anemia (12.5%), neutropenia (9.4%), pneumonia (9.4%), and myalgia (9.4%).
Reference
- Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantlecell lymphoma. Blood Adv. 2021;5(12):2577-2585. doi:10.1182/bloodadvances.2020004074
Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.