Reassessments of ESAs for Cancer Treatment in the US and Europe
Anemia is a widely prevalent complication among cancer patients. At the time of diagnosis, 30% to 40% of patients with non-Hodgkin lymphoma or Hodgkin lymphoma and up to 70% of patients with multiple myeloma are anemic; rates are higher among persons with myelodysplastic syndromes. Among patients with solid cancers or lymphomas, up to half develop anemia following chemotherapy. For almost 2 decades, erythropoiesis-stimulating agents (ESAs) were the primary treatment for cancer-related anemia. However, reassessments of benefits and risks of ESAs for cancer-associated anemia have occurred internationally. We reviewed guidelines and notifications from regulatory agencies and manufacturers, reimbursement policies, and utilization for ESAs in the cancer and chronic kidney disease settings within the United States, Europe, and Canada. In 2008 the US Food and Drug Administration (FDA) restricted ESAs from cancer patients seeking cure. Reimbursement is limited to hemoglobin levels < 10 g/dL. In the United States, ESA usage increased 340% between 2001 and 2006, and decreased 60% since 2007. The European Medicines Agency (EMEA) recommended that ESA benefits do not outweigh risks. In Europe between 2001 and 2006, ESA use increased 51%; since 2006, use decreased by 10%. In 2009, Canadian manufacturers recommended usage based on patient preferences. In Canada in 2007, approximately 20% of anemic cancer patients received ESAs, a 20% increase since 2004. In contrast to Europe, where ESA use has increased over time, reassessments of ESA-associated safety concerns in the United States have resulted in marked decrements in ESA use among cancer patients.
Anemia is a widely prevalent complication among cancer patients. At the time of diagnosis, 30% to 40% of patients with non-Hodgkin lymphoma or Hodgkin lymphoma and up to 70% of patients with multiple myeloma are anemic; rates are higher among persons with myelodysplastic syndromes. Among patients with solid cancers or lymphomas, up to half develop anemia following chemotherapy. For almost 2 decades, erythropoiesis-stimulating agents (ESAs) were the primary treatment for cancer-related anemia. However, reassessments of benefits and risks of ESAs for cancer-associated anemia have occurred internationally. We reviewed guidelines and notifications from regulatory agencies and manufacturers, reimbursement policies, and utilization for ESAs in the cancer and chronic kidney disease settings within the United States, Europe, and Canada. In 2008 the US Food and Drug Administration (FDA) restricted ESAs from cancer patients seeking cure. Reimbursement is limited to hemoglobin levels < 10 g/dL. In the United States, ESA usage increased 340% between 2001 and 2006, and decreased 60% since 2007. The European Medicines Agency (EMEA) recommended that ESA benefits do not outweigh risks. In Europe between 2001 and 2006, ESA use increased 51%; since 2006, use decreased by 10%. In 2009, Canadian manufacturers recommended usage based on patient preferences. In Canada in 2007, approximately 20% of anemic cancer patients received ESAs, a 20% increase since 2004. In contrast to Europe, where ESA use has increased over time, reassessments of ESA-associated safety concerns in the United States have resulted in marked decrements in ESA use among cancer patients.
Anemia is a widely prevalent complication among cancer patients. At the time of diagnosis, 30% to 40% of patients with non-Hodgkin lymphoma or Hodgkin lymphoma and up to 70% of patients with multiple myeloma are anemic; rates are higher among persons with myelodysplastic syndromes. Among patients with solid cancers, up to half develop anemia following chemotherapy or radiochemotherapy.[1,2]
The pathophysiology of tumor-associated anemia is multifactorial. Among persons with hematologic malignancies, bone marrow involvement with malignant cells can result in progressive anemia. Other causes include iron or vitamin deficiencies, occult bleeding, autoimmune hemolysis or pure red blood cell aplasia, or an anemia of chronic disease. Manifestation and severity of anemia vary among patients. Mild to moderate anemia can cause headache, palpitations, tachycardia, and shortness of breath. Chronic anemia can lead to severe organ damage affecting the cardiovascular system, immune system, lungs, kidneys, muscles, and the central nervous system.[3] There is ongoing debate about the impact of cancer-related anemia on quality of life (QOL).
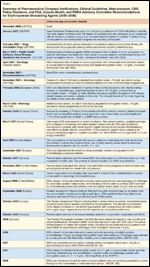
TABLE 1
Summary of Pharmaceutical Company Notifications, Clinical Guidelines, Meta-analyses, CMS Policy Decisions, and FDA, Canada Health, and EMEA Advisory Committee Recommendations for Erythropoisesis-Stimulating Agents (2006-2008)
Another aspect of anemia in patients with malignant disease is the effects of anemia on tumors. For Hodgkin disease, chronic lymphocytic leukemia, cervical carcinoma, and cancer of the head and neck, anemia is an independent prognostic factor.[4] Anemia and associated increased tumor hypoxia might result in a poorer response to radiotherapy or chemotherapy.[5] Severe anemia may result in dose reduction or delay of chemotherapy, leading to higher tumor burden and decreased survival.[6] These observations generated the hypothesis that strategies to diminish cancer-related anemia might also improve tumor response and extend survival.
Historically, blood transfusion was the conventional treatment of choice for severe cancer-related anemia. Although homologous blood transfusion is the fastest method of alleviating symptoms, potential short- and long-term risks include transmission of infectious diseases, transfusion-associated allergic reactions, alloimmunization, overtransfusion, immune modulation with possible adverse effects on tumor growth, and even death resulting from major incompatibilities.
Recombinant human erythropoietin is a treatment option for cancer-related anemia. Human erythropoietin is an acidic glycoprotein hormone. Approximately 90% of the hormone is synthesized in the kidney and 10% in the liver.[7] Tissue hypoxia is the most important trigger for increased synthesis. Basal production maintains a relatively constant plasma concentration of erythropoietin in vivo, within a range from 9 to 26 mU/mL. Effects of erythropoietin in the bone marrow are mediated by a specific surface receptor mainly located on erythroid progenitor and precursor cells.[8] Two functions of erythropoietin include stimulating proliferation of progenitor cells and maintaining their viability.[9] Three forms of erythropoiesis-stimulating agents (ESAs) are available for use-recombinant human erythropoietin (rHuEPO-α [Epogen, Procrit]), rHuEPO-β (only outside the United States), and darbepoetin-α (Aranesp), a long-acting erythropoietin preparation.
The agent rHuEPO was first approved for the treatment of anemia in chronic kidney failure. In 1990, erythropoietin was introduced in cancer therapy regimens for patients with multiple myeloma. A pilot study showed hematologic response rates of 85% and an improved performance status.[10] Adverse effects such as hypertension, headaches, and thrombotic events conclusively attributable to erythropoietin treatment were reported in very few patients.[10] In 1993, randomized, placebo-controlled trials in anemic cancer patients demonstrated that rHuEPO resulted in a significant reduction in transfusion requirements. Based on these trials, recombinant erythropoietin was approved by the US Food and Drug Administration (FDA) to decrease transfusion requirements among anemic patients with nonmyeloid malignancies with chemotherapy-associated anemia not due to other reversible causes. Initially, incorporation of rHuEPO into practice was limited in part by physician perception that mild-to-moderate anemia among cancer patients was asymptomatic and did not warrant intervention. In 2001, three open-label trials of rHuEPO in the community setting in anemic patients receiving cancer chemotherapy identified decreased transfusion requirements. A European randomized, placebo-controlled trial by Littlewood et al confirmed these results and suggested improved survival with rHuEPO treatment.[11]
In 2006, a Cochrane Review included 27 randomized controlled studies on erythropoietin with 3,287 adults, published between 1985 and April 2002.[12] The review reported that erythropoietin decreases the risk of receiving red blood cell transfusions and augments the risk (likelihood) of achieving a hematologic response (as defined by a transfusion-independent hemoglobin rise of 2 g/dL). On average, participants in the ESA group received one less unit of blood than the control group. There was inconclusive evidence as to whether ESAs improved tumor response and overall survival. There were no statistically significant adverse effects. Furthermore, evidence was inconclusive with respect to fatigue reduction and to QOL.
Shortly after the Cochrane Review database was locked, concern was raised regarding the impact of ESAs on survival and thromboembolic events. Two 2003 studies in cancer patients reported increased mortality in patients treated with ESAs.[13,14] Following a May 2004 Oncologic Drugs Advisory Committee (ODAC) hearing, the FDA concluded that the hemoglobin target for ESA treatment should not be higher than 12 g/dL. Package inserts in the United States were subsequently amended to include this recommendation.
Because erythropoietin receptors have been detected in numerous cancers,[15] it is also possible that endogenously produced or exogenously administered erythropoietin promotes the proliferation and survival of erythropoietin receptor–expressing cancer cells.[16] There is an ongoing debate about the validity of these studies. Other researchers have postulated an antiapoptotic effect of ESAs on other tissues including neural and cancer cells.[17] In addition, there may be a link between endogenous erythropoietin and angiogenesis in vivo.[18] Possibly, endogenous erythropoietin is needed to promote tumor angiogenesis and to maintain the viability of endothelial cells. However, clinical implications of these findings have not been clarified. Apart from the direct tumor growth stimulation, a pathophysiologic relationship between thromboembolic events and cancer has been described. Studies have implicated tumor-mediated activation of the hemostatic system in the formation of tumor stroma and in tumor metastasis.[19]
With these findings as background, the subject of ESAs in the oncology setting has been extensively reevaluated in the United States, Canada, and Europe in recent years. Herein, we briefly summarize these materials.
Current Regulatory-Approved Oncology Indications
In the United States, ESAs are indicated to avoid transfusions. For chemotherapy-associated anemia, no specific trigger or target hemoglobin level is included in the label. In Europe, ESAs are indicated for treating symptomatic chemotherapy-associated anemia or chronic kidney disease–associated anemia, with target hemoglobin levels between 10 and 12 g/dL.[20]
Safety
Eight clinical trials identified mortality and tumor progression risks when patients with non-Hodgkin lymphoma or cancer of the head and neck, cervix, breast, or lung received ESAs. One study reported a 1.3-fold increase in relative risk of death when patients not receiving active therapy received ESAs.[21] Meta-analyses identified 1.10- to 1.19-fold higher relative mortality risks and 1.57- to 1.7-fold increased relative venous thromboembolic event (VTE) risks when cancer patients receive ESAs.[22,23]
Advisory Committees
FDA statisticians concluded that QOL claims by manufacturers for cancer patients were not statistically valid.[24] The FDA’s ODAC initially warned against administering ESAs to breast, head and neck, and non–small-cell lung cancer patients because of tumor-promotion concerns.[25] Later, ODAC recommended against ESA administration with potentially curative chemotherapy.
The Committee for Medicinal Products for Human Use (CHMP), a scientific committee of the European Medicines Agency (EMEA), concluded that ESA benefits outweighed risks[20]; symptomatic patients with chemotherapy-associated anemia should receive ESAs targeted to hemoglobin levels between 10 and 12 g/dL. Subsequently, CHMP warned of increased mortality among ESA-treated cancer patients and stated that for cancer patients with long life expectancies, ESA benefits do not outweigh tumor progression and mortality risks. The EMEA’s Scientific Advisory Group on Oncology concluded that ESA decisions should be based on risk-benefit assessments and patient preference.
Manufacturer Notifications
Black Box warnings from 2007 describe VTE, cardiovascular, and mortality risks with ESA treatment of chemotherapy-associated anemia to achieve target hemoglobin levels > 12 g/dL and mortality risks with ESA administration if no chemotherapy is administered.[26] Another revision identified cancer progression/mortality risks among lymphoid and non–small-cell lung cancer patients receiving ESAs targeted to hemoglobin levels > 12 g/dL. Subsequently, clinicians were warned against administering ESAs to patients receiving potentially curative myelosuppressive therapy; for patients receiving palliative chemotherapy, clinicians were cautioned to withhold ESAs if hemoglobin levels needed to avoid transfusion were exceeded.[27]
In the United States, manufacturers have developed patient medication guides warning that ESAs can cause death or other serious side effects and that serious heart problems, including heart attack, stroke, heart failure, and early death, may occur if patients receive ESAs at hemoglobin levels
> 12 g/dL.[28] The medication guides also note that blood clots may occur with ESAs; that ESAs do not improve symptoms of anemia, QOL, fatigue, or well-being; and that tumors may grow faster and patients die sooner when ESAs are used to raise hemoglobin levels beyond levels necessary to avoid transfusions or are given to patients who are not getting strong chemotherapy doses.[28] It is not known whether these risks exist when ESAs are given according to FDA-
approved-use directions.
In Europe, manufacturers disseminated Public Assessment Reports (PARs) warning clinicians and patients against achieving hemoglobin levels > 12 g/dL and recommending that ESA decisions be based on assessments of risk, benefit, and patient preferences. Revised PARs warned of increased death risks with ESA administration to advanced head and neck cancer patients receiving radiation therapy, metastatic breast cancer patients receiving chemotherapy, and patients with active malignant disease who are not receiving therapy.[29]
Guidelines/Technology Assessments
TABLE 2

Current Guidelines and Package Inserts for Erythropoisesis-Stimulating Agent Use in the Cancer Setting in the United States, Canada, and Europe
National Comprehensive Cancer Network guidelines advise that ESAs are indicated to prevent transfusions among cancer patients receiving palliative chemotherapy; no trigger/target hemoglobin levels are reported.[30]
The Working Party of the European Organisation for Research and Treatment of Cancer guidelines stated that ESAs reduced transfusions, improved QOL, and should be initiated at hemoglobin levels of 9 to 11 g/dL in symptomatic cancer patients receiving chemotherapy and considered in asymptomatic chemotherapy patients with hemoglobin levels of 11 to 11.9 g/dL.[31]
Reimbursement
In the United States in 2007, the United States Pharmacopeia removed anemia of cancer as a covered indication. The Centers for Medicare & Medicaid Services (CMS) restricts anemia reimbursement secondary to chemotherapy for solid tumors, multiple myeloma, lymphoma, and chronic lymphocytic leukemia. The guidance limits initiation of ESA use to patients with hemoglobin < 10 g/dL, duration of use up to 8 weeks following final doses of myelosuppressive chemotherapy, and target hemoglobin levels < 10 g/dL. In Europe, ESA reimbursement is included in global provider payments.
Utilization
Patterns of use of ESAs in the oncology setting have changed dramatically in the United States, and less so in Europe.[32-36] In the United States, prior to 2007, ESAs were administered to 67% of cancer patients with anemia. Beginning in 2007, ESA utilization decreased markedly among persons with cancer not receiving chemotherapy, after the FDA advised that a phase III trial found higher death rates and no reduction in transfusions with darbepoetin vs placebo. Utilization further declined when CMS withheld ESA reimbursement among chemotherapy-associated anemia patients with hemoglobin levels > 10 g/dL. Previously, 50% of ESA use for chemotherapy-associated anemia was at hemoglobin levels > 10 g/dL and hemoglobin levels were usually maintained at > 10 g/dL. ESA use declined by 18% to 55%, while transfusions increased by 2% to 6%. Another decline in use occurred in 2008, following recommendations that ESAs should not be administered to anemic cancer patients receiving potentially curative therapy and warnings in patient medication guides of ESA-associated risks of death and other serious adverse events.
One study reported ESA use decreased 78% between 2006 and 2008, while transfusion rates increased 3%.[32] Another identified 14% increased transfusion rates between 2007 and 2008.[33] A third study reported ESA use decreased to 33% among anemic cancer patients, with most of these individuals being anemic cancer patients receiving palliative chemotherapy.[34] ESA usage increased 340% between 2001 and 2006 and subsequently decreased by 60%, attributed to infrequent ESA use among cancer patients receiving potentially curative chemotherapy and among patients receiving palliative chemotherapy.[35] ESA administration now targets hemoglobin levels < 10 g/dL. In Europe for cancer, ESA use increased from 17% with hemoglobin levels < 12 g/dL in 2001 to 62% with hemoglobin levels < 11 g/dL in 2007.[36] ESA use in France, Germany, Italy, Spain, and the United Kingdom increased 220% during these years. In 2008, ESA use for chemotherapy-associated anemia decreased 10%; patients were informed of thromboembolism risks but not tumor progression/mortality risks, and physicians were advised to administer ESAs based on risk-benefit considerations and patient preferences.
Implementing New Risk Assessments
In concert with its expanded powers under Title IX of the FDA Amendments Act (FDAAA) of 2007,[37] the FDA recently approved the class-wide Risk Evaluation and Mitigation Strategy (REMS) program for ESAs.[38] This REMS will require physicians and hospitals using ESAs for cancer-related anemia to register and undergo training regarding the risks and benefits of these drugs in order to continue prescribing them in the cancer setting. The FDAAA, which went into effect on March 25, 2008, provides a new statutory framework and authority for the FDA to require REMS for drugs and biologics either prior to approval if the FDA determines that it is “necessary to ensure that the benefits of the drug outweigh the risks of the drug,” (FDC Act § 505-1(a)(1)), or after drug approval if the FDA “becomes aware of new safety information and makes a determination that such a strategy is necessary to ensure that the benefits of the drug outweigh the risks of the drug” (FDC Act § 505-1(a)(2)).[37]
The ESA REMS, which does not require patients to register, consists of a medication guide, communication plan, and elements to assure safe use (ETASU).[38] The ETASU includes certification for healthcare providers who both prescribe and dispense ESAs, as well as the hospitals themselves. Furthermore, it requires that certified hospitals and healthcare providers dispense ESAs only after they have discussed the risks with the patient and the patient has signed an Acknowledgment Form.[38] The required medication guide, explaining the risks and benefits of ESAs, is to be provided to all affected patients seen at retail/hospital outpatient pharmacies as well as physician offices, clinics, inpatient hospital and outpatient clinics, and upon patient request.
In tandem with REMS is the establishment of the Assisting Providers and Cancer Patients with Risk Information for the Safe Use of ESAs (APPRISE) program to mitigate the risk of decreased survival and/or poorer tumor outcomes in patients with cancer.[38] While APPRISE contains information specific only to the oncology setting, the FDA mandates that all patients taking ESAs (renal and cancer patients) receive a medication guide. Hospitals and physicians are also provided with an enrollment number and must reenroll in APPRISE every 3 years. Mechanisms are in place to ensure compliance, with the penalty for noncompliance being suspension of access to ESAs for implicated healthcare facilities. The ESA REMS program will begin on March 24, 2010. Once REMS is fully implemented, ESAs can only be ordered for cancer patients by facilities and practitioners in full program compliance. The FDA will convene a specific advisory panel meeting in late 2010 to discuss use of ESAs in the nephrology setting.
Conclusions
For cancer, package inserts in the United States indicate that ESAs are associated with death risks and warn that ESAs are not indicated for anemia treatment among patients receiving potentially curative chemotherapy nor among patients not receiving active therapy. Also, the CMS National Coverage Decision limits reimbursement to chemotherapy-associated anemia with hemoglobin < 10 mg/dL. The result is 60% decreased ESA use since 2006, with 33% of anemic cancer patients now receiving ESAs (primarily with palliative chemotherapy) and target hemoglobin levels < 10 g/dL. In Europe and Canada, labels warn that ESA-treated patients with chemotherapy-associated anemia should be evaluated for patient preferences and risk-benefits. Patient information states that ESAs reduce transfusion needs, while side effects include headache, hypertension, blood clots, joint pain,

and edema. Currently, 62% of patients with chemotherapy-associated anemia in Europe receive ESAs, with target hemoglobin levels between 10 and 12 g/dL. In Canada, where ESA-financial allotments are limited, ESA use occurs in 20% of patients with chemotherapy-associated anemia and ESA use increased by 30% since 2001.
Varying interpretations of risks and benefits of ESAs internationally are now apparent. The United States provides the most severe warnings for use and has experienced marked decreases in ESA use for cancer patients. In contrast, ESA administration for cancer patients has increased over time in Europe, and safety warnings are less onerous. It is certain that the situation will continue to be fluid, and updates on safety and efficacy will be reported at frequent intervals.
Financial Disclosure:Dr. Bennett has served as a consultant and has received grant support from Amgen previously. Dr. Silver is a consultant for the Gerson Lehrman Group.
Acknowledgement:This manuscript was supported by funding from grants received by the RADAR (Research on Adverse Drug events and Reports) project from the National Cancer Institute (1R01CA 102713-01 and P 30 CA60553). JM McKoy received support from the National Cancer Institute through a Mentored Career Development Award (1 K01 CA134554-01). SY Lai received support from the Flight Attendant Medical Research Institute Young Clinical Scientist Award and a National Institutes of Health Mentored Career Development Award (K08 DE018061).
References:
References
1. Ludwig H, Sundal E, Pecherstorfer M, et al: Recombinant human erythropoietin for the correction of cancer associated anemia with and without concomitant cytotoxic chemotherapy. Cancer 76:2319-2329, 1995.
2. Ludwig H, Fritz E: Anemia of cancer patients: Patient selection and patient stratification for epoetin treatment. Semin Oncol 25(suppl 7):35-38, 1998.
3. Nissenson AR: Epoetin and cognitive function. Am J Kidney Dis 20:21-24, 1992.
4. Nowrousian MR: Pathophysiology of cancer-related anemia, in Nowrousian MR (ed): Recombinant Human Erythropoietin (rhEPO) in Clinical Oncology, pp 39-62. Wien–New York, Springer, 2002.
5. Hockel M, Knoop C, Schlenger K, et al: Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 26:45-50, 1993.
6. Knocke TH, Weitmann HD, Feldmann HJ, et al: Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother Oncol 53(2):99-104, 1999.
7. Koury ST, Bondurant MC, Koury MJ: Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 71:524-527, 1988.
8. Spivak JL: Cancer-related anemia: Its causes and characteristics. Semin Oncol 21(suppl 3):3-8, 1994.
9. Koury MJ, Bondurant MC: Control of red cell production: The roles of programmed cell death (apoptosis) and erythropoietin. Transfusion 30:673-674, 1990.
10. Abels R: Erythropoietin for anemia in cancer patients. Eur J Cancer 29A(suppl 2):2-8, 1993.
11. Littlewood TJ, Bajetta E, Nortier JW, et al: Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: Results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 19:2865-2874, 2001.
12. Bohlius J, Wilson J, Seidenfeld J, et al: Recombinant human erythropoietins and cancer patients: Updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 98:708-714, 2006.
13. Leyland-Jones B: Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 4:459-460, 2003.
14. Henke M, Laszig R, Rube C, et al: Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 362:1255-1260, 2003.
15. Arcasoy MO, Jiang X, Haroon ZA: Expression of erythropoietin receptor splice variants in human cancer. Biochem Biophys Res Commun 307:999-1007, 2003.
16. Henke M, Mattern D, Pepe M, et al: Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol 24:4708-4713, 2006 (see erratum in J Clin Oncol 25:1457, 2007).
17. Um M, Gross AW, Lodish HF: A "classical" homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal 19:634-645, 2007.
18. Hardee ME, Cao Y, Fu P, et al: Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS One 2:e549, 2007.
19. Levine MN, Lee AY, Kakkar AK: From Trousseau to targeted therapy: New insights and innovations in thrombosis and cancer. J Thromb Haemost 1:1456-1463, 2003.
20. European Medicines Agency: Public Statement: Epoetins and the risk of tumour growth progression and thromboembolic events in cancer patients and cardiovascular risks in patients with chronic kidney disease. October 2007. Available at http://www.emea.europa.eu/pdfs/human/press/pus/49618807en.pdf. Accessed February 11, 2010.
21. Smith RE Jr, Aapro MS, Ludwig H, et al: Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: Results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 26:1040-1050, 2008.
22. Bennett CL, Silver SM, Djulbegovic B, et al: Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 299:914-924, 2008.
23. Bohlius J, Schmidlin K, Brillant C, et al: Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 373:1532-1542, 2009 (see erratum in Lancet 374: 28, 2009).
24. US Food and Drug Administration: Erythropoeisis-stimulating agents (ESAs) [Changes to Procrit prescribing information], March 9, 2007. Available at http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm129253.htm. Accessed February 16, 2010.
25. US Food and Drug Administration Center for Drug Evaluation and Research: FDA Advisory Committee Briefing Document: Reassessment of the Risks of Erythropoiesis-Stimulating Agents (ESAs) Administered for the Treatment of Anemia Associated with Chronic Renal Failure. Presented at the Joint meeting of the Cardiovascular and Renal Drugs Advisory Committee and the Drug Safety and Risk Management Committee, September 11, 2007. Available at http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4315b1-01-FDA.pdf. Accessed February 11, 2010.
26. Epogen [package insert]. Amgen, Inc. Thousand Oaks, CA, November 2007. Approved by the US Food and Drug Administration November 8, 2007. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103234s5158lbl.pdf. Accessed February 12, 2010.
27. Epogen [package insert labeling changes]. Amgen, Inc. Thousand Oaks, CA, August 2008. Approved by the US Food and Drug Administration November 19, 2008. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103234s5195sPI.pdf. Accessed February 12, 2010.
28. Amgen: Patient Medication Guide: Epogen (epoetin alfa). August 2008. Approved by the US Food and Drug Administration November 19, 2008. Available at http://www.fda.gov/downloads/Drugs/DrugSafety/ucm088591.pdf. Accessed February 12, 2010.
29. European Medicines Agency: Assessment Report for Abseamed. London, October 23, 2008. Doc. ref. no. EMEA/672835/2008. Available at http://www.ema.europa.eu/humandocs/PDFs/EPAR/abseamed/Abseamed-H-727-II-06-AR.pdf. Accessed February 12, 2010.
30. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Cancer and Chemotherapy–induced Anemia. V.1.2009. Available at http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf. Accessed February 11, 2010.
31. Bokemeyer C, Aapro MS, Courdi A, et al: EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 43:258-270, 2007.
32. Amgen 2007 Annual Report and Financial Summary. Available at http://phx.corporate-ir.net/External.File?item=UGFyZW50SUQ9MjAxMTJ8Q2hpbGRJRD0tMXxUeXBlPTM=&t=1. Accessed February 12, 2010. Amgen, Inc., Thousand Oaks, CA.
33. Naeim A, Glaspy J: Changes in RBC supportive medications and transfusions in cancer patients undergoing chemotherapy before and after FDA and Medicare actions in 2007 (abstract 20595). J Clin Oncol 26(May 20 suppl), 2008.
34. Vadhan-Raj S HV, Zhou X, Sizer K, et al: Impact of safety concerns of erythropoiesis-stimulating agents (ESAs) and regulatory changes on the use of ESAs and red blood cell (RBC) transfusions at a comprehensive cancer center (abstract 1300). Presented at the 50th American Society of Hematology Annual Meeting and Exposition; December 6, 2008; San Francisco, CA.
35. Hess G, Nordyke RJ, Pirolli M, et al: Effect of changes in labeling and reimbursement on use of ESAs and transfusions (abstract 20589). J Clin Oncol 26(suppl 15S), 2008.
36. Ludwig H, Aapro M, Bokemeyer C, et al: Treatment patterns and outcomes in management of anaemia in cancer patients in Europe: Findings from the Anaemia Cancer Treatment Act (ACT) study. Eur J Cancer 45:1603-1615, 2009.
37. US Food and Drug Adminstration Amendments Act of 2007. Available at http://frwebgate.access.gpo.gov/cgibin/getdoc.cgi?dbname=110_cong_public_laws&docid=f:publ085.110. Accessed on March 2, 2010.
38. US Food and Drug Administration: Safety Announcement: Information on Erythropoiesis-Stimulating Agents (ESA) Epoetin alfa (marketed as Procrit, Epogen) Darbepoetin alfa (marketed as Aranesp). February 16, 2010. Available at http://www.fda.gov/drugs/drugsafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109375.htm. Accessed on March 2, 2010.