33 Real-world Patient Characteristics, Treatment Patterns, and Outcomes Among Patients With Metastatic Breast Cancer and BRCA Mutations Treated Off-label With Talazoparib
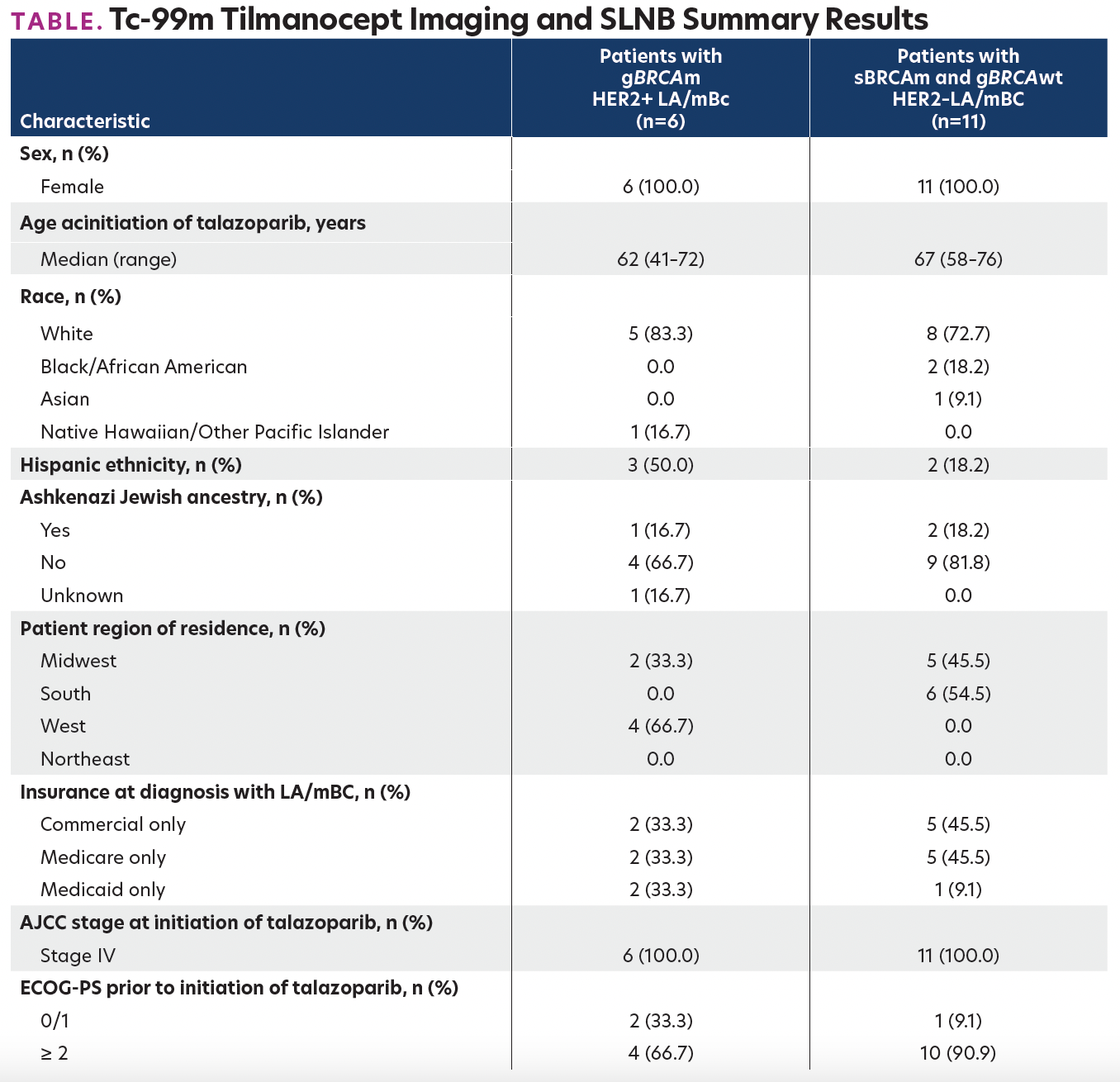
TABLE. Tc-99m Tilmanocept Imaging and SLNB Summary Results
Background
Talazoparib is approved by the US FDA for the treatment of adult patients (pts) with germline BRCA-mutated (gBRCAm) HER2-negative (HER2–) locally advanced or metastatic breast cancer (mBC). This exploratory analysis describes the pt characteristics, treatment patterns, and outcomes among two rare groups of pts treated off-label with talazoparib in the real-world setting in the US: (1) pts with gBRCAm HER2-positive (HER2+) mBC and (2) pts with HER2– mBC with somatic BRCA-mutated (sBRCAm) and gBRCA wild-type (gBRCAwt) mBC.
Materials and Methods
Physicians from the Cardinal Health Oncology Provider Extended Network abstracted data from the medical records of US adult pts who initiated talazoparib as monotherapy or as combination therapy on or after 10/16/18 and had (1) gBRCAm HER2+ mBC; or (2) sBRCAm and gBRCAwt HER2– mBC. The Kaplan-Meier method was used to describe time-to-event outcomes.
Results
Six talazoparib-treated women with gBRCAm HER2+ mBC and 11 women with sBRCAm and gBRCAwt HER2– mBC treated in community oncology practices were included (Table). Among pts with gBRCAm HER2+ mBC, talazoparib was most often administered as 4th-line therapy. The median real-world progression-free survival (rwPFS) for talazoparib was 10.0 months, and the overall response rate (ORR) during talazoparib treatment was 83%. Among pts with sBRCAm and gBRCAwt HER2– mBC, talazoparib was most often initiated as 2nd line therapy. The median rwPFS was 3.9 months, and the ORR was 9%.
Conclusions
Findings from this study describe clinical outcomes among a small number of pts with BRCA-mutated mBC treated off-label with talazoparib.
AFFILIATIONS:
Reshma L. Mahtani,1* Jasmina Ivanova,2 Angelica Falkenstein,3 Alexander Niyazov,2 Joanne C. Ryan,2 Jonathan Kish,3 Ajeet Gajra,3 Parisa Asgarisabet,3 Kristin M. Zimmerman Savill3
1Miami Cancer Institute, Miami, FL.
2Pfizer Inc, New York, NY.
3Cardinal Health Specialty Solutions, Cardinal Health, Dublin, OH.
*Presenting author
