49 Financial Toxicity Among Patients With Breast Cancer During the COVID-19 Pandemic
TABLE
Background
Treatment for cancer poses a significant financial burden to patients. Patients with breast cancer (BC) are at particular risk for more financial hardship, given the long natural history of BC and the need for longitudinal multidisciplinary care. Financial toxicity (FT)—defined as the negative financial distress experienced by patients related to cancer treatment—is increasingly recognized as an adverse outcome of cancer care. FT encompasses the objective financial consequences of treatment costs and subjective financial concerns. The “toxicity” of cancer-related financial burden has been associated with worse quality of life, treatment nonadherence, and higher mortality. The COVID-19 pandemic has exacerbated financial stress among cancer patients, including increased debt and difficulty paying for health care and non–health care needs. While there is an increasing body of research exploring FT and its impact on patients, data on FT among patients with BC during COVID-19 are limited.
Materials and Methods
This cross-sectional study of 669 patients with BC was recruited from the Ciitizen platform, breastcancer.org, and patient advocacy groups between March 30 and July 6, 2021. Data on sociodemographic and clinical characteristics, FT, and COVID-19 diagnosis history were collected via an online self-reported survey. FT was assessed with the validated COmprehensive Score for financial Toxicity (COST) instrument. Higher COST scores indicate lower levels of FT.
Results
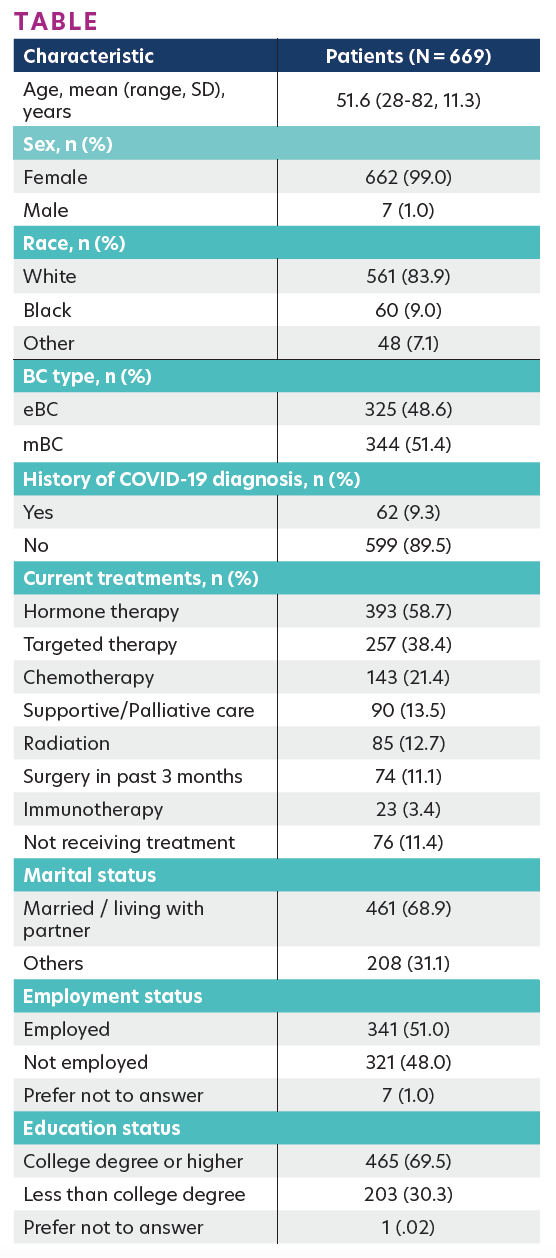
Of the 669 patients, the mean age was 51.6 years (range, 28–82), 83.9% were White, 99.0% were female, and 51.4% had metastatic BC (mBC). A total of 62 (9.3%) reported ever being diagnosed with COVID-19. The mean ± SD COST score was 22.7±10.8. The COST scores were significantly higher in patients with early BC (eBC) than mBC (24.2±11.3 vs 21.3±10.2; P <.001). COST scores did not significantly differ by COVID-19 diagnosis history (21.3±9.8 vs 22.9±10.9; P = .263). Using the FT grading cutoff, patients with mBC were more likely to experience FT (no FT, 33.7%; mild FT, 42.2%; moderate/severe FT, 24.1%) than patients with eBC (no FT, 48.3%; mild FT, 31.1%; moderate/ severe FT, 20.6%; P ≤ .001). No significant differences in the prevalence of FT were observed across racial groups or between patients with/without COVID diagnosis history. The Table shows key patient characteristics and FT.
Conclusions
Financial toxicity was common in patients with BC during COVID-19, particularly among those with mBC. The findings highlight the need to better understand financial burden to improve the quality of life in patients with mBC.
AFFILIATIONS:
Yan Wu,1,2 Xianchen Liu,2 Martine C. Maculaitis,3 Benjamin Li,2 Alexa Berk,4 Angelina Massa,4 Marisa C. Weiss,5 Lynn McRoy2
1Rutgers University, Piscataway, NJ.
2Pfizer Inc, New York, NY.
3Cerner Enviza, Malvern, PA.
4Invitae Corporation, San Francisco, CA.
5Breastcancer.org, Ardmore, PA.
