53 Comparison of Surgical Complications With Direct-to-Implant vs Tissue Expander Reconstruction After Wise Pattern Skin-Sparing Mastectomy
53 Comparison of Surgical Complications With Direct-to-Implant vs Tissue Expander Reconstruction After Wise Pattern Skin-Sparing Mastectomy

Background
Wise Pattern Mastectomy is a common incision utilized in patients with large, ptotic breasts undergoing skin-sparing mastectomy and immediate breast reconstruction (IBR). This incision pattern is associated with an increased risk of delayed wound healing and skin necrosis, which may be further influenced by the type of IBR performed. We compared surgical complications in patients undergoing IBR with Direct-to-Implant (DTI) vs Tissue Expander (TE) after Wise Pattern Skin-Sparing Mastectomy (WSSM).
Materials and Methods
Patients who underwent WSSM and IBR from 2019 to 2023 were selected. Patient characteristics, clinical features, and surgical complications were compared between patients who underwent DTI vs TE IBR. Multivariable logistic regression analysis was performed to identify factors associated with major complications (surgical site infection [SSI], skin necrosis requiring reoperation, and reconstruction loss) and any 30-day complication controlling for patient age, race, ethnicity, body mass index (BMI), presence of diabetes, tobacco use, neoadjuvant chemotherapy, reason for mastectomy, axillary surgery, mastectomy weight, and type of reconstruction.
Status
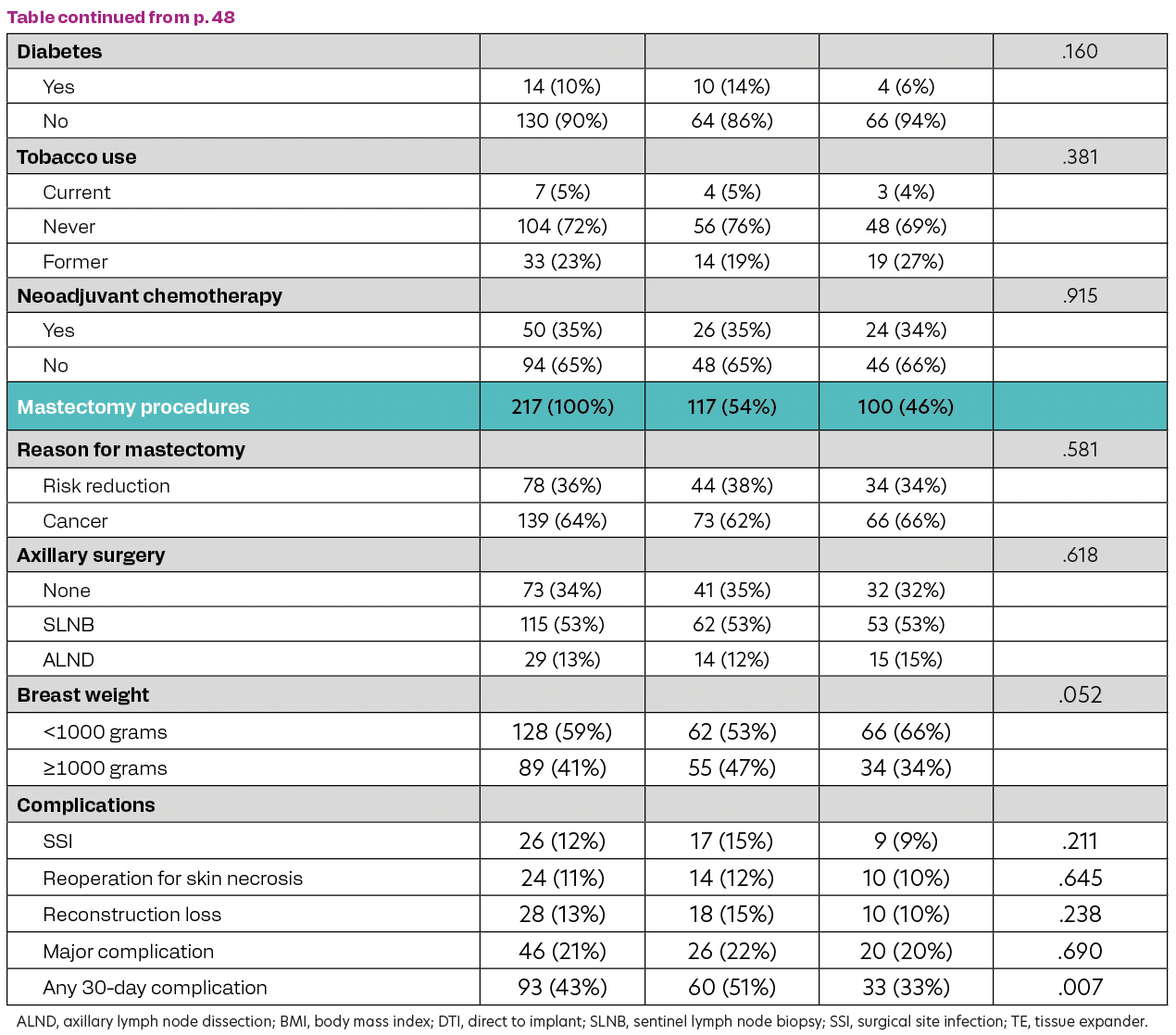
A total of 144 patients who underwent 217 mastectomies were evaluated: 73 bilateral (51%) and 71 unilateral (49%); 117 DTI (54%) and 100 TE (46%). (Table) Most patients were ≥ 50 years old (64%), White (83%), Hispanic (64%), and had a BMI < 30 kg/m2 (58%). Neoadjuvant chemotherapy was utilized in 35% of patients. The reason for mastectomy was cancer in 64%, and axillary surgery was performed in 66% of cases. The mastectomy weight was ≥ 1000 grams in 41% of cases. Major complications occurred in 21% of cases: SSI in 12% (DTI 15% vs TE 9%), skin necrosis requiring reoperation in 11% (DTI 12% vs TE 10%), and reconstruction loss in 13% (DTI 15% vs TE 10%). SSI and skin necrosis requiring reoperation were associated with reconstruction loss (SSI, P ≤.001; skin necrosis, P ≤.001). Multivariable analysis showed that breast weight ≥ 1000 grams was associated with major complications (OR, 2.82; 95% CI 1.27-6.26; P = .011) and Hispanic ethnicity, current smoking, and DTI reconstruction were associated with any 30-day complication (Hispanic ethnicity: OR, 3.33; 95% CI 1.62-6.87; P = .001; current smoking: OR, 5.57; 95% CI 1.05-29.02; P = .044; DTI, OR, 2.40; 95% CI 1.31-4.40; P = .005).

Table. Patient Characteristics, Clinical Features, and Surgical Outcomes