54 The Treatment of Breast Cancer With Percutaneous Thermal Ablation: Results of the THERMAC Trial
54 The Treatment of Breast Cancer With Percutaneous Thermal Ablation: Results of the THERMAC Trial

Background/Significance
Percutaneous thermal ablation has the potential to replace surgical treatment and improve the health-related quality of life of patients with small breast cancers, without jeopardizing current treatment effectiveness or safety. The objective of this study was to determine the efficacy rate in terms of complete ablation for the most promising techniques of thermal ablation: radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation (CA) for patients with early-stage breast cancer to warrant a randomized, phase 3 trial comparing thermal ablation with surgery.
Materials and Methods
This was an investigator-initiated, randomized, phase 2 “pick a winner” study in postmenopausal patients with cT1N0 estrogen receptor–positive, HER2-negative breast cancer. Patients were randomly assigned to undergo RFA, MWA or CA, and underwent surgical resection 3 months after thermal ablation. The optimal technique should meet the minimum requirements of a complete ablation rate of at least 85%, a complication rate of ≤ 15%, and completion of the treatment without interruption due to pain or discomfort in at least 80% of patients. The primary outcome was proportion of patients with complete pathological response at pathology examination; secondary outcomes included ability to complete the thermal ablative procedure in an outpatient setting and the occurrence of adverse events (AEs).
Results
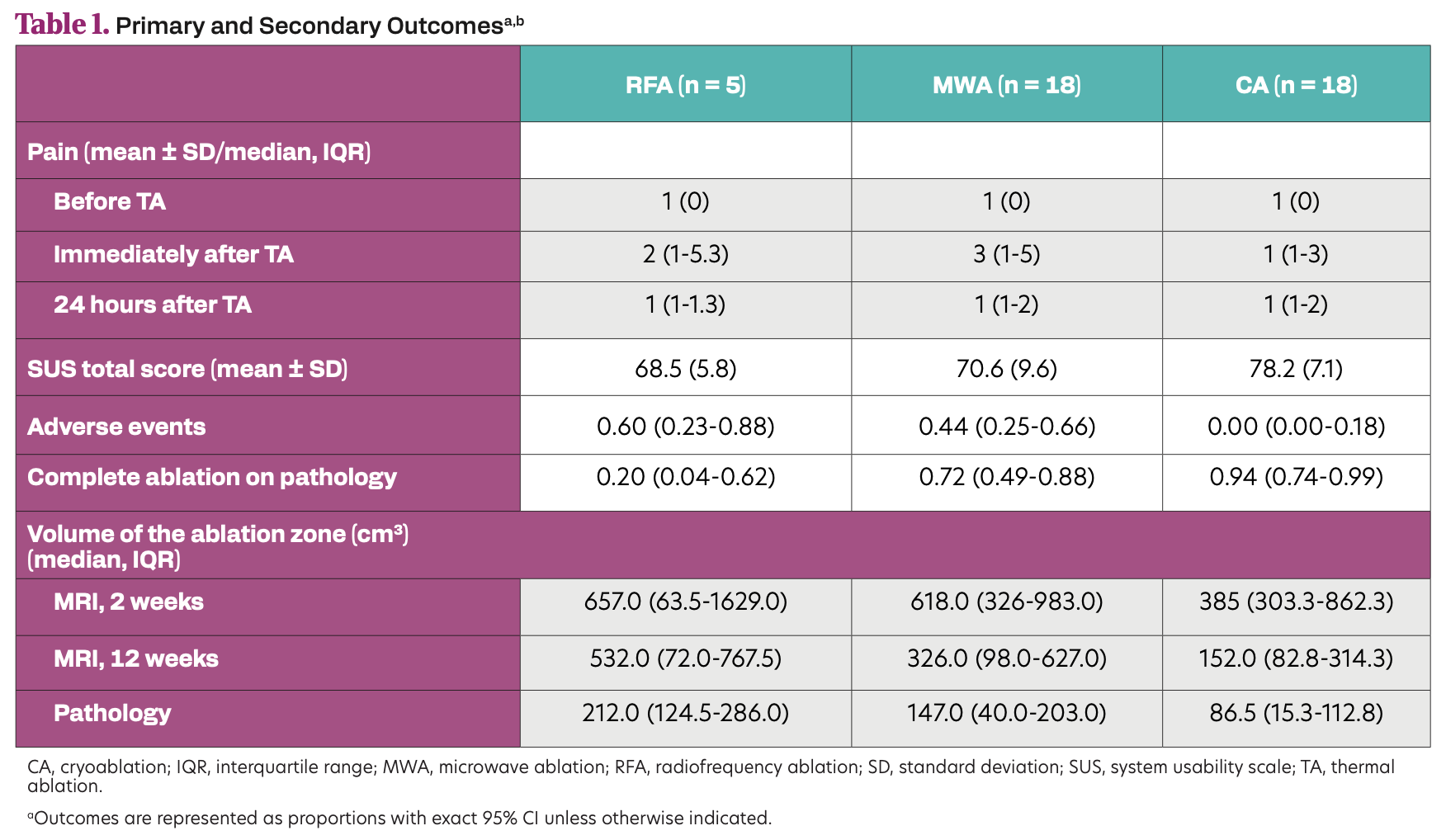
A total of 41 patients were included. Treatment in the RFA arm was terminated prematurely due to reaching a protocol defined stopping rule after 5 inclusions. Eighteen patients were treated with MWA and CA. All techniques were user-friendly and showed excellent feasibility in an outpatient setting. Complete ablation was reached in 72% (95% CI, 49%-88%) in the MWA arm and in 94% (95% CI, 74%-99%) in the CA arm. AEs occurred in 44% (95% CI, 25%-66%) and 0% (95% CI, 0%-18%) of patients in the MWA and CA arms, respectively.
Table 1. Primary and Secondary Outcomesa,b
Conclusion
Cryoablation was the only thermal ablative technique that met the minimum requirements and will therefore be selected for the phase 3 trial.
