A Phase 2 Study of Docetaxel and Capecitabine in Advanced Squamous Cell Carcinoma of the Head and Neck
This study explores the efficacy and safety of combining docetaxel and capecitabine for treating recurrent or metastatic head and neck cancer.
Introduction
Cancer of the head and neck refers to a collection of cancers, predominantly squamous cell carcinoma, that occur in various sites, including the oral cavity, pharynx, larynx, nasal cavity, paranasal sinuses, and salivary glands. In 2024, oral and pharyngeal cancers accounted for an estimated 4% of newly diagnosed cancers in men in the US, making them the eighth most common malignancy.1 At the time of diagnosis, 10% of head and neck cancer cases will present as metastatic disease.2 In addition, the development of metastatic disease is estimated to be approximately 20% to 30% in patients who present with earlier-stage disease.2 Patients who present with advanced-stage disease that is unresectable have a 5-year survival rate of 30% as compared with 60% to 98% 5-year survival rates in patients who present with earlier-stage disease.3 Results of a recent study showed that the 1-year survival rate for conventional head and neck squamous cell carcinoma (HNSCC) is 36.5%.4
With recent advances in the management of locally advanced HNSCC and better locoregional control, the proportion of patients with distant metastases appears to be increasing as compared with those with locoregional relapse.5,6 Thus, evaluating newer treatments of metastatic disease has become increasingly important. Therapy usually involves a platinum-based regimen. In the past, cisplatin or carboplatin has been combined with 5-fluorouracil (5-FU) to achieve response rates of 20% to 30% with overall survival (OS) of approximately 5 to 6.6 months.7 The addition of the anti-EGFR antibody cetuximab in the phase 3 EXTREME trial (NCT00122460) improved median OS to 10 months.8 Recently, checkpoint inhibitors (CPIs) were evaluated and have become part of the standard-of-care regimens.9-12
Although the recent data appear promising in the management of metastatic disease, the problem with platinum-based therapy is related to its toxicity. In addition, once a cancer stops responding to immunotherapy agents, treatment will be restricted to chemotherapy drugs, which are limited. Hence, studies evaluating newer and safer therapeutic strategies are needed to improve outcomes. The NCCN currently lists several regimens for first-line or subsequent treatments, such as a combination of chemotherapy with immunotherapy; a combination of chemotherapy with cetuximab; and chemotherapy, either as a combination (platinum with taxanes or platinum with 5-FU or capecitabine) or monotherapy, such as docetaxel alone or capecitabine alone.13
Preclinical studies in human cancer xenograft models showed that taxanes such as docetaxel and paclitaxel upregulate thymidine phosphorylase in tumor tissues.14 More importantly, coadministration of docetaxel or paclitaxel with capecitabine resulted in synergistic tumor cell killing in a xenograft model.14
The combination of docetaxel and capecitabine has been studied in different solid tumors with reasonable efficacy and safety. O’Shaughnessy et al conducted a study that included women with metastatic breast cancer. In that study, the combination of docetaxel (75 mg/m2) and capecitabine (1250 mg/m2) resulted in significantly improved progression-free survival (PFS) and OS when compared with docetaxel alone.15 In another randomized phase 3 study that included patients with pretreated metastatic breast cancer, the combination of capecitabine (1250 mg/m2) and docetaxel (75 mg/m2) was found to have a response rate of 32%.16 In a prostate cancer study of weekly docetaxel (35 mg/m2) and capecitabine (650 mg/m2), the response rate was 68.2%, and 87% of patients completed 4 cycles of therapy.17 The combination of weekly docetaxel and capecitabine was found to have an OS similar to data seen with the cisplatin/5-FU regimen.18
Because both docetaxel and 5-FU are active agents in squamous cell carcinoma of the head and neck, we aimed to evaluate the efficacy and the safety of the combination of docetaxel and capecitabine in patients with recurrent or metastatic HNSCC.
Methods
Study design and participants
This was a phase 2 open-label, nonrandomized trial for patients with recurrent or metastatic HNSCC. The study was conducted at the University of Nebraska Medical Center (UNMC) Fred & Pamela Buffett Cancer Center and affiliated clinics. Patients were eligible for enrollment if they were 19 years or older; had histologically proven HNSCC (except for nasopharyngeal or salivary gland tumors) that was recurrent or metastatic and not curable by local therapy; had a Karnofsky Performance Status score of 7 or higher; and had no prior chemotherapy for metastatic HNSCC. Full inclusion criteria are included in the protocol (supplementary file). The study protocol was approved by the Institutional Board Review at UNMC and the appropriate scientific research and ethics committees. The study was done in accordance with the protocol. All participants provided written informed consent before enrollment.
Procedures
The treatment included docetaxel 75 mg/m2 intravenously on
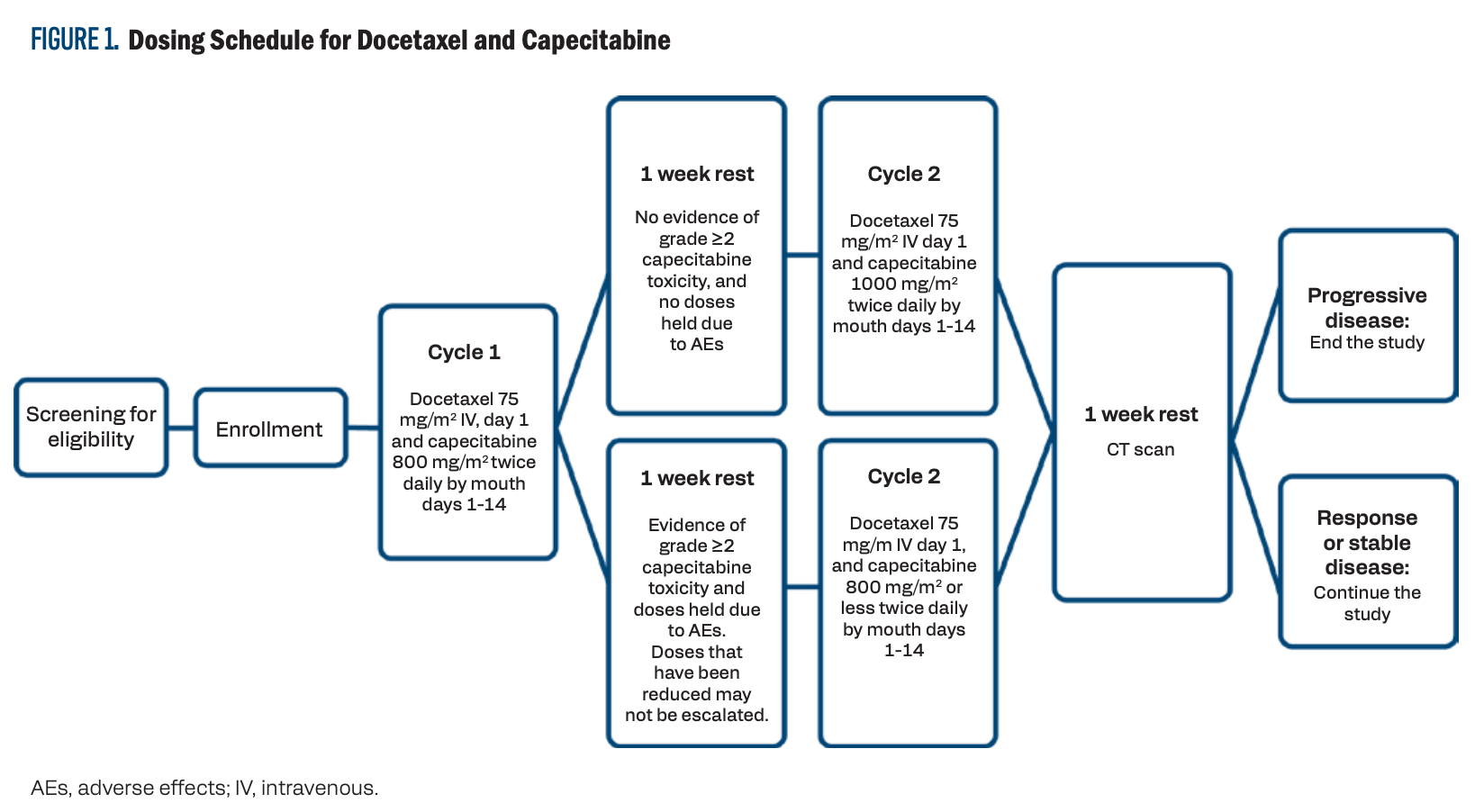
day 1 and capecitabine 800 mg/m2 orally twice daily, on days 1 to 14 of a 3-week cycle. If there was no evidence of grade 2 or greater toxicity with capecitabine, and if the doses of capecitabine were not held due to adverse effects (AEs) after the first cycle, then the dose of capecitabine was increased to 1000 mg/m2 twice daily on days 1 to 14 for the remaining cycles. Response assessment with CT imaging was done after every 2 cycles per standard of care and at the completion of the study. Participants with stable or responsive disease continued therapy until progression (Figure 1).
FIGURE 1. Dosing Schedule for Docetaxel and Capecitabine
Outcomes
The primary end point was the objective response rate (ORR), defined as complete response (CR) or partial response (PR) rates as defined by RECIST criteria at 14 weeks.19 Secondary end points included PFS, OS, incidence and severity of AEs, and quality of life.
Statistical methods
This phase 2 study utilized a Simon optimal 2-stage design with a planned first-stage sample size of 18.20 If 2 evaluable participants or fewer achieved a CR or PR at week 15, the study would be terminated. If 3 or more evaluable participants achieved a CR or PR, 25 additional participants would be accrued in the second stage, for a total of 43 evaluable participants.
The plan was to complete the study only if it had a response rate of at least 25%. The optimal 2-stage design was to test the null hypothesis that the CR or PR is 0.10 or lower vs that CR or PR rate is 0.25 or higher at the significance level of 0.05 and with power of 0.80.
Efficacy and safety were analyzed in all participants who received at least 1 treatment cycle. ORR was descriptively summarized using percentages and 95% CIs. The Kaplan-Meier method was used to estimate time-to-event distributions for PFS and OS. AEs were summarized using participant-level incidence rates.
Results
This trial was terminated early due to CPIs being introduced as standard of care.A total of 14 patients enrolled in the study. The median age of the study population was 66 years. Patients’ demographics are summarized in Table 1.
TABLE 1. Patient Characteristics
Efficacy
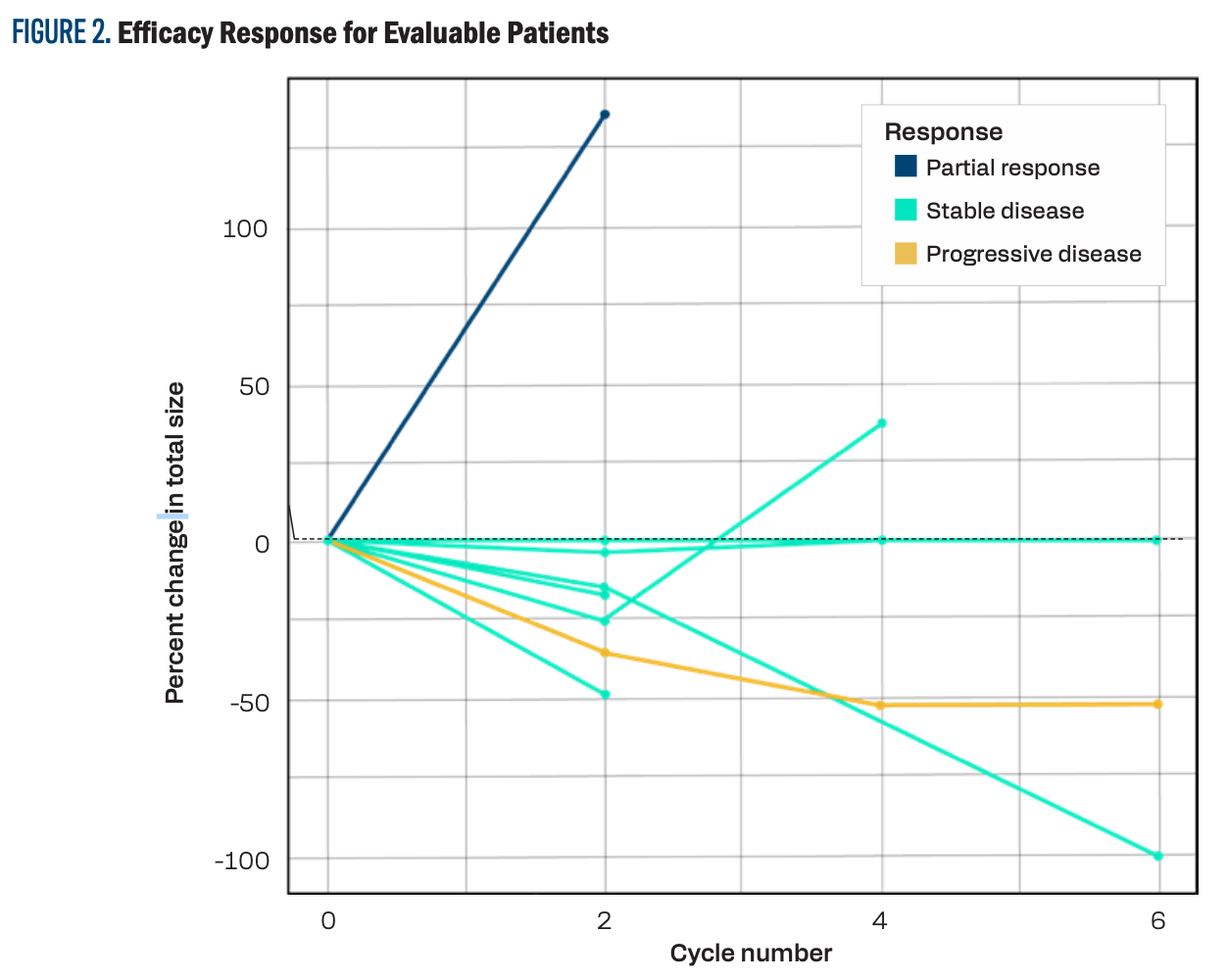
Only 9 patients were evaluable in the study. One patient achieved a PR, 6 patients had stable disease, none achieved a CR, and 2 developed progressive disease. Five patients were not evaluable. The combination had an ORR of 11% and a disease stability rate (DSR) of 77.7%. Figure 2 shows the individual response for evaluable patients.
FIGURE 2. Efficacy Response for Evaluable Patients
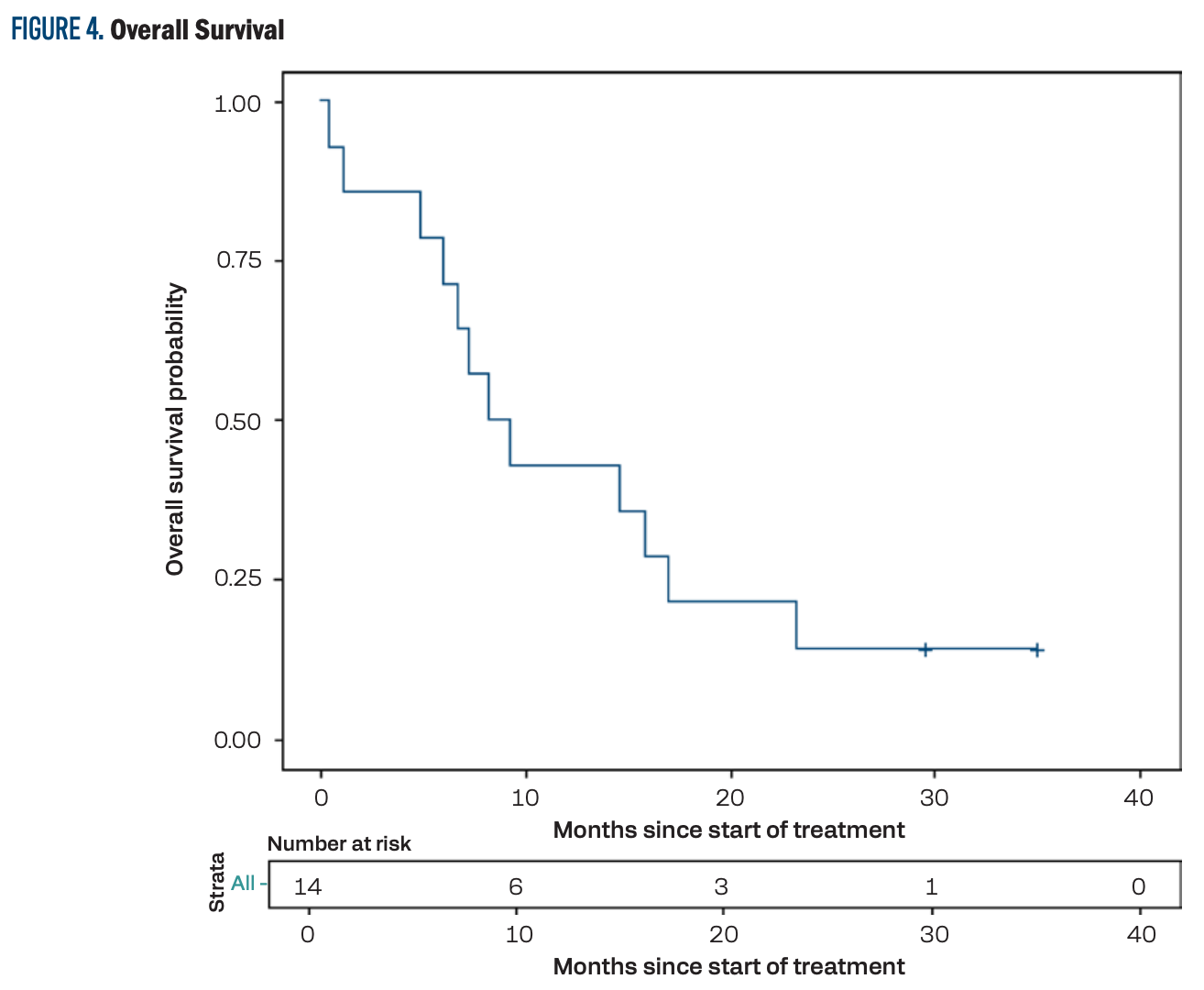
The median PFS was 4.9 months (95% CI, 1.1-8.2; Figure 3), and the median OS was 8.7 months (95% CI, 4.8-17.0; Figure 4). The PFS at 12 months was 14.3% (95% CI, 2.3%-36.6%) and the OS at 12 months was 42.9% (95% CI, 17.7%-66.0%).
FIGURE 3. Progression-Free Survival
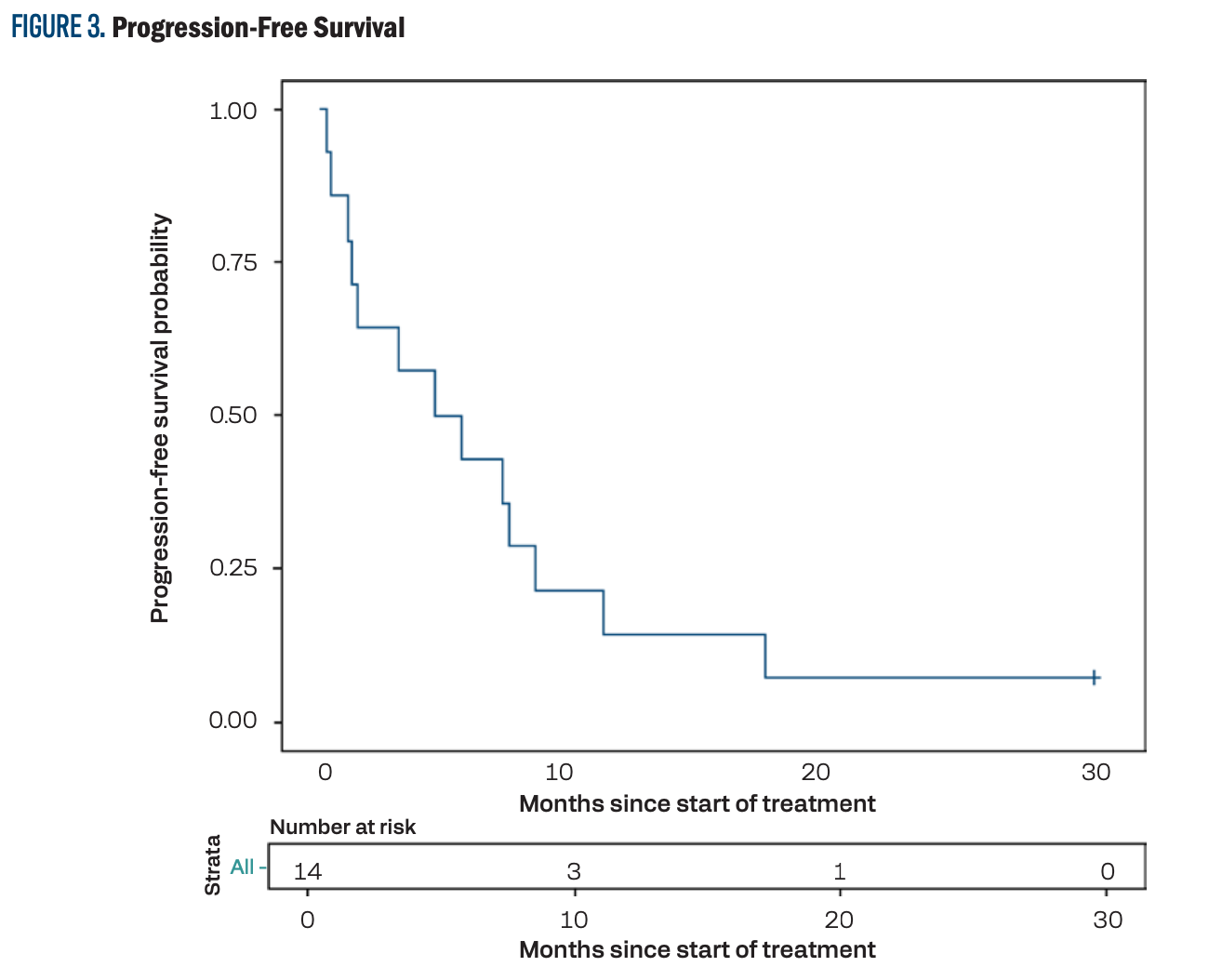
FIGURE 4. Overall Survival
Safety
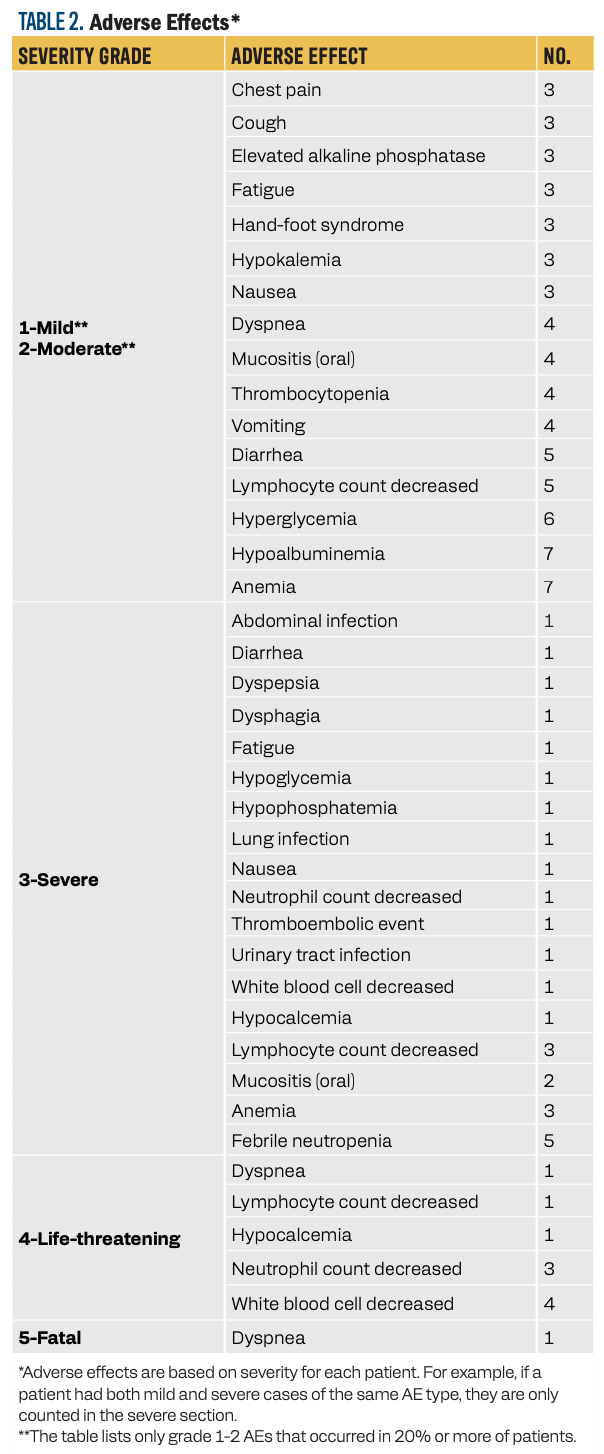
One fatal event occurred during treatment, which was related to dyspnea and acute respiratory failure and was thought to be unrelated to treatment. Hematologic toxicity was the most common grade 3 or greater AE. Febrile neutropenia occurred in 36% of patients. In our study, granulocyte colony-stimulating factor was not used as primary prophylaxis.
It is worth noting that we did not observe any grade 3 or greater hand-foot syndrome (HFS) reactions with this combination; however, HFS did occur in 3 patients (21%; grades 1/2). The most common (defined as ≥ 20%) grade 1/2 AEs and all grades 3 to 5 AEs are summarized in Table 2.
TABLE 2. Adverse Effects*
Discussion
This phase 2 trial evaluated the combination of docetaxel with capecitabine in the setting of recurrent and refractory HNSCC. It is the first trial that has used this combination in patients with HNSCC. However, it was terminated early due to the change in the standard of care with the introduction of CPIs. This was based on several studies, including the phase 3 CheckMate 141 trial (NCT02105636), which studied nivolumab in recurrent HNSCC.21 This study showed an improved OS in the nivolumab arm compared with the standard-of-care arm that included single-agent systemic therapy of methotrexate, docetaxel, or cetuximab (7.5 months vs 5.1 months, respectively).
The phase 3 KEYNOTE-048 trial (NCT02358031) evaluated pembrolizumab in untreated locally incurable recurrent or metastatic HNSCC.9,10 The study had 3 arms: pembrolizumab alone, pembrolizumab with platinum therapy and 5-FU, and cetuximab with platinum therapy and 5-FU. This study showed that improvement in OS reached 14.7 months in a certain population based on PD-L1 expression, whether pembrolizumab was used with chemotherapy or as a monotherapy. This has led to different trials, such as the phase 4 KEYNOTE-B10 trial (NCT04489888), which evaluated pembrolizumab with platinum therapy and paclitaxel. It showed a median OS of 13.1 months.11,12 Pembrolizumab was also evaluated as a single agent in recurrent and refractory HNSCC, and it demonstrated both safety and efficacy in that setting.22-24 Thus, CPI became a standard of care in the first-line setting, and utilizing it in the second-line setting became rare, leaving patients with just chemotherapy options, which gives this trial importance again.
Preclinical studies in human cancer xenograft models have shown that docetaxel and paclitaxel further upregulate thymidine phosphorylase in tumor tissues, and coadministration with capecitabine has resulted in synergistic tumor cell killing in a xenograft model.14 A study evaluated different schedules for the combination of docetaxel and capecitabine in different human cancer xenograft models, including docetaxel-sensitive MX-1 and MAXF401 human mammary, A755 murine mammary, and the less–docetaxel-sensitive WiDr human colon xenograft.25 The combination included capecitabine given orally for 2 weeks, with docetaxel given intravenously in a 3-week regimen on day 1, 8, or 15. The combination showed superior efficacy compared with either docetaxel or capecitabine in all xenografts. However, in the breast tumor models, docetaxel administered on day 8 had higher antitumor activity than when administered on days 1 or 15. In contrast, in the colon cancer xenograft, docetaxel administered on day 1 demonstrated higher antitumor activity compared with other days of administration.25
Current evidence suggests that if the 2 agents are used in combination, docetaxel should be started at a higher dose, and capecitabine should be started at a dose lower than its maximum tolerated dose. If AEs develop, the docetaxel dose should be decreased.26
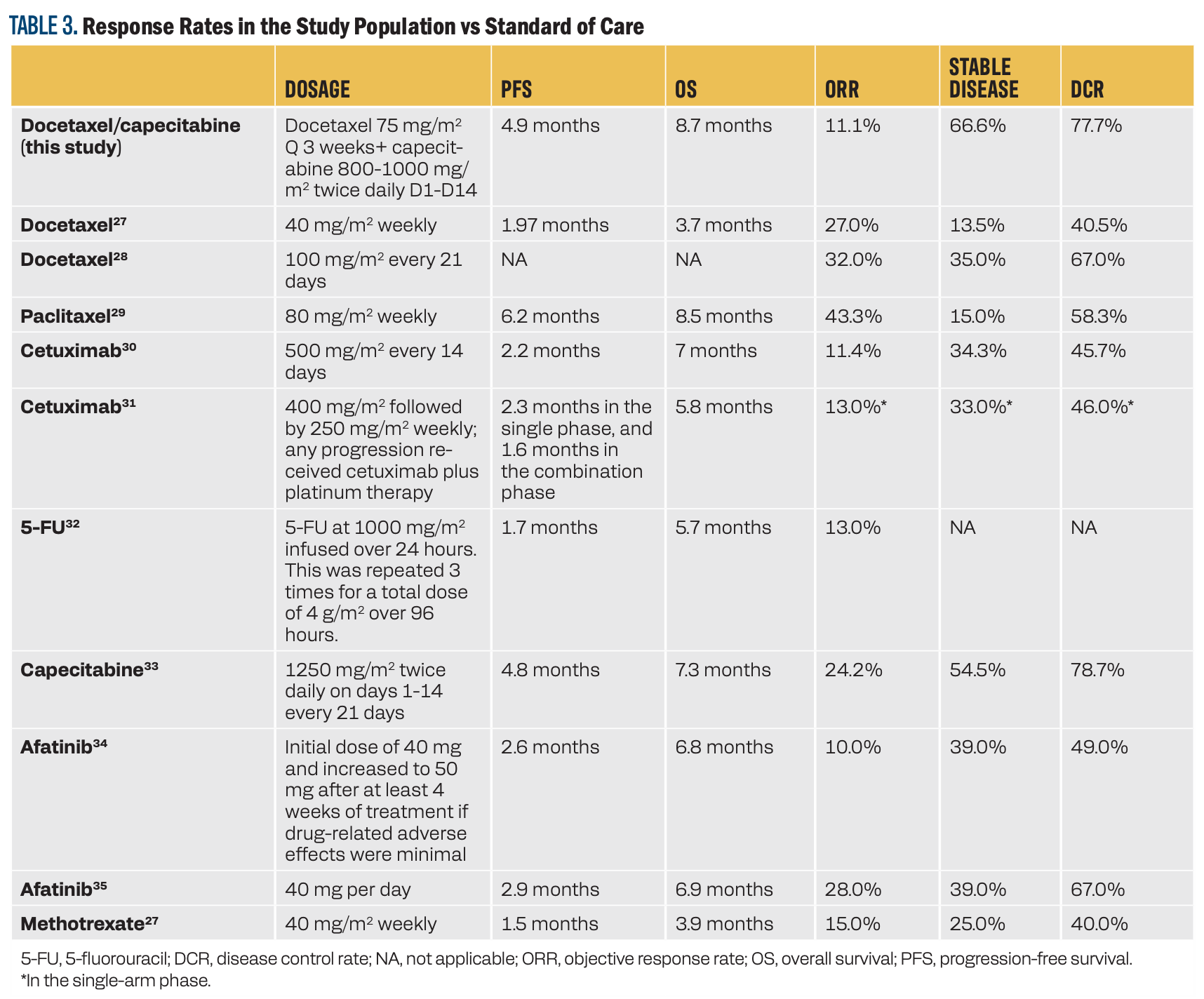
In this study, the combination of docetaxel and capecitabine resulted in a 77.7% DSR and 11% ORR. In addition, it led to a PFS of 4.9 months and an OS of 8.7 months. Table 3 compares the response rates, PFS, and OS of this combination vs the current standard-of-care, second-line, and third-line treatment options.
TABLE 3. Response Rates in the Study Population vs Standard of Care
This study had an acceptable safety profile and a low incidence of HFS. Although no additional supportive care measures were implemented beyond the standard of care, we believe the low percentage of severe toxicity observed in the study can be attributed to starting capecitabine at a lower dose. This is particularly relevant, given that most patients with HNSCC typically have a history of mucositis and damage from previous treatments.
Conclusion
The combination of docetaxel and capecitabine led to a good disease response rate in this study. Despite the study’s early termination due to changes in standard of care, this combination can be considered an option for treatment in patients with advanced HNSCC, especially in patients who progress following immunotherapy or are not candidates for immunotherapy.
Corresponding author
Omar K. Abughanimeh, MBBS
Address: 986840 Nebraska Medical Center,
Omaha, NE 68198-6840
Email: omar.abughanimeh@unmc.edu
Phone number: +1 (402) 559-8500
Fax number: +1 (402) 559-6520
Acknowledgment
The author team would like to thank all our patients who participated in the clinical trial, and the research team at the Fred & Pamela Buffett Cancer Center and peripheral sites who participated in the trial.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Pisani P, Airoldi M, Allais A, et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol Ital. 2020;40(suppl1):S1-S86. doi:10.14639/0392-100X-suppl.1-40-2020
- Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184-194. doi:10.1056/NEJM199301213280306
- Takenaka Y, Uno A, Tanaka H, Takemoto N, Inohara H. Distant metastasis in head and neck squamous cell carcinoma variants: a population-based study. Head Neck. 2023;45(4):882-889. doi:10.1002/hed.27305
- Vikram B, Strong EW, Shah JP, Spiro R. Failure at distant sites following multimodality treatment for advanced head and neck cancer. Head Neck Surg. 1984;6(3):730-733. doi:10.1002/hed.2890060305
- Yao M, Lu M, Savvides PS, et al. Distant metastases in head-and-neck squamous cell carcinoma treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(2):684-689. doi:10.1016/j.ijrobp.2011.07.014
- Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1245-1251. doi:10.1200/JCO.1992.10.8.1245
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116-1127. doi:10.1056/NEJMoa0802656
- Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi:10.1016/S0140-6736(19)32591-7
- Burtness B, Rischin D, Greil R, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022;40(21):2321-2332. doi:10.1200/JCO.21.02198
- Dzienis M, Cundom J, Fuentes CS, et al. Pembrolizumab plus carboplatin and paclitaxel as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (KEYNOTE-B10): a single-arm phase IV trial. J Clin Oncol. 2024;42(25):2989-2999. doi:10.1200/JCO.23.02625
- Black CM, Zheng D, Hair GM, et al. Real-world use of first-line pembrolizumab + platinum + taxane combination regimens in recurrent / metastatic head and neck squamous cell carcinoma. Front Oncol. 2024;14:1348045. doi:10.3389/fonc.2024.1348045
- NCCN. Clinical Practice Guidelines in Oncology. Head and neck cancers, version 4.2025. Accessed May 30, 2025. https://tinyurl.com/mpsu9tzd
- Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res.1998;4(4):1013-1019.
- O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20(12):2812-2823. doi:10.1200/JCO.2002.09.002
- Chan S, Romieu G, Huober J, et al. Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol. 2009;27(11):1753-1760. doi:10.1200/JCO.2007.15.8485
- Ferrero JM, Chamorey E, Oudard S, et al. Phase II trial evaluating a docetaxel-capecitabine combination as treatment for hormone-refractory prostate cancer. Cancer. 2006;107(4):738-745. doi:10.1002/cncr.22070
- Zhang J, Lee J, Urba S, Worden F. A phase II trial evaluating weekly docetaxel and capecitabine in patients with metastatic or advanced, locally recurrent head and neck cancers. Cancer Invest. 2010;28(9):910-916. doi:10.3109/07357907.2010.483502
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi:10.1016/j.ejca.2008.10.026
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1-10. doi:10.1016/0197-2456(89)90015-9
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi:10.1056/NEJMoa1602252
- Chow LQ, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838-3845. doi:10.1200/JCO.2016.68.1478
- Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi:10.1016/S1470-2045(16)30066-3
- Cohen EEW, Soulières D, Le Tourneau C, et al; KEYNOTE-040 Investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156-167. doi:10.1016/S0140-6736(18)31999-8
- Fujimoto-Ouchi K, Tanaka Y, Tominaga T. Schedule dependency of antitumor activity in combination therapy with capecitabine/5’-deoxy-5-fluorouridine and docetaxel in breast cancer models. Clin Cancer Res. 2001;7(4):1079-1086.
- Frances N, Woloch C, Marouani H, Mercier C, Iliadis A. Optimize administration protocol of capecitabine plus docetaxel combination in metastatic breast cancer patients. Curr Top Med Chem. 2012;12(15):1665-1668. doi:10.2174/156802612803531306
- Guardiola E, Peyrade F, Chaigneau L, et al. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer. 2004;40(14):2071-2076. doi:10.1016/j.ejca.2004.05.019.
- Catimel G, Verweij J, Mattijssen V, et al. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Ann Oncol. 1994;5(6):533-537. doi:10.1093/oxfordjournals.annonc.a058908
- Grau JJ, Caballero M, Verger E, Monzó M, Blanch JL. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol. 2009;129(11):1294-1299. doi:10.3109/00016480802590451
- Fury MG, Sherman E, Lisa D, et al. A randomized phase II study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancer. J Natl Compr Canc Netw. 2012;10(11):1391-1398. doi:10.6004/jnccn.2012.0144
- Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171-2177. doi:10.1200/JCO.2006.06.7447
- Jacobs C, Lyman G, Velez-García E, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10(2):257-263. doi:10.1200/JCO.1992.10.2.257
- Martinez-Trufero J, Isla D, Adansa JC, et al. Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. Br J Cancer. 2010;102(12):1687-1691. doi:10.1038/sj.bjc.6605697
- Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583-594. doi:10.1016/S1470-2045(15)70124-5
- Guo Y, Ahn MJ, Chan A, et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): an open-label, randomised phase III trial. Ann Oncol. 2019;30(11):1831-1839. doi:10.1093/annonc/mdz388

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.